Robocath04.09.19
Robocath, a company that designs, develops and commercializes cardiovascular robotic systems for the treatment of vascular diseases, has obtained CE certification for its R-One robotic device. As a result, it can begin commercialization in Europe and the Middle East.
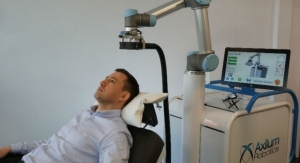
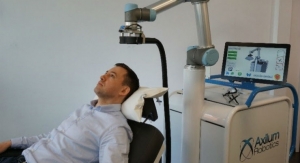
R-One is the first European robotic solution to obtain the CE mark in the field of interventional cardiology. The robot assists interventional cardiologists in stenting (angioplasty) by enabling precision technologies that complement existing methods, in order to improve procedures and the working environment.
To achieve its objectives, Robocath is planning a new fundraising campaign, with the support of its historical shareholders, for the commercialization of its device over the next few years.
Philippe Bencteux, M.D., chairman and founder of Robocath, said: “The whole Robocath team is proud to have taken this major step forward in our development, which comes after our first sale of a training robot to the Medical Training & Testing Center in Rouen. Our entry into the market for interventional vascular robotics represents a substantial opportunity for growth. There is the potential for the R-One solution to be installed in more than 3,000 procedure rooms, performing around 1.6 million interventions each year in Europe alone. Building on our CE mark, we expect to finalize a number of strategic distribution partnerships very shortly in high potential geographic areas, with a Europe and Middle-East wide launch expected in 2020.”
Founded in 2009 by Bencteux, Robocath designs, develops, and commercializes robotic solutions to treat cardiovascular diseases. As an active player in the evolving medical robotic industry, these solutions aim to make medical procedures safer, thanks to reliable technologies, while complementing manual interventions.
R-One is the first solution developed by Robocath. It uses a technology that optimizes the safety of robotic-assisted coronary angioplasty. This medical procedure consists of revascularizing the cardiac muscle by inserting one or more implants (stents) into the arteries that supply it with blood. Every 30 seconds, somewhere in the world, this type of procedure is performed. R-One is designed to operate with precision and perform specific movements creating better interventional conditions. Thanks to its open architecture, R-One is compatible with market leading devices and cath labs.
Robocath aims to become the world leader in vascular robotics and develop the remote treatment of vascular emergencies, guaranteeing the best care pathway for all. Based in Rouen, Robocath has more than 20 employees and is financially supported by regional investment funds (NCI, Normandy participations, GO CAPITAL), national investment funds (M Capital, Supernova Invest), by several business angels and financial institutions (Caisse d'épargne, BNP Paribas, Crédit Agricole) and Bpifrance.
R-One is the first European robotic solution to obtain the CE mark in the field of interventional cardiology. The robot assists interventional cardiologists in stenting (angioplasty) by enabling precision technologies that complement existing methods, in order to improve procedures and the working environment.
To achieve its objectives, Robocath is planning a new fundraising campaign, with the support of its historical shareholders, for the commercialization of its device over the next few years.
Philippe Bencteux, M.D., chairman and founder of Robocath, said: “The whole Robocath team is proud to have taken this major step forward in our development, which comes after our first sale of a training robot to the Medical Training & Testing Center in Rouen. Our entry into the market for interventional vascular robotics represents a substantial opportunity for growth. There is the potential for the R-One solution to be installed in more than 3,000 procedure rooms, performing around 1.6 million interventions each year in Europe alone. Building on our CE mark, we expect to finalize a number of strategic distribution partnerships very shortly in high potential geographic areas, with a Europe and Middle-East wide launch expected in 2020.”
Founded in 2009 by Bencteux, Robocath designs, develops, and commercializes robotic solutions to treat cardiovascular diseases. As an active player in the evolving medical robotic industry, these solutions aim to make medical procedures safer, thanks to reliable technologies, while complementing manual interventions.
R-One is the first solution developed by Robocath. It uses a technology that optimizes the safety of robotic-assisted coronary angioplasty. This medical procedure consists of revascularizing the cardiac muscle by inserting one or more implants (stents) into the arteries that supply it with blood. Every 30 seconds, somewhere in the world, this type of procedure is performed. R-One is designed to operate with precision and perform specific movements creating better interventional conditions. Thanks to its open architecture, R-One is compatible with market leading devices and cath labs.
Robocath aims to become the world leader in vascular robotics and develop the remote treatment of vascular emergencies, guaranteeing the best care pathway for all. Based in Rouen, Robocath has more than 20 employees and is financially supported by regional investment funds (NCI, Normandy participations, GO CAPITAL), national investment funds (M Capital, Supernova Invest), by several business angels and financial institutions (Caisse d'épargne, BNP Paribas, Crédit Agricole) and Bpifrance.