Maria Shepherd, President and Founder, Medi-Vantage04.03.19
Transcatheter aortic valve replacement (TAVR), once the last resort for patients so sick or elderly they were deemed unable to outlive surgical aortic valve replacement performed via open-heart surgery, has now been proven to offer benefits for younger, low-risk patients, based on the findings from two large clinical trials (Table 1). The first was published in The New England Journal of Medicine (Popma, et. al.),1 and the second—the Partner ll trial2—was sponsored by Edwards Lifesciences.
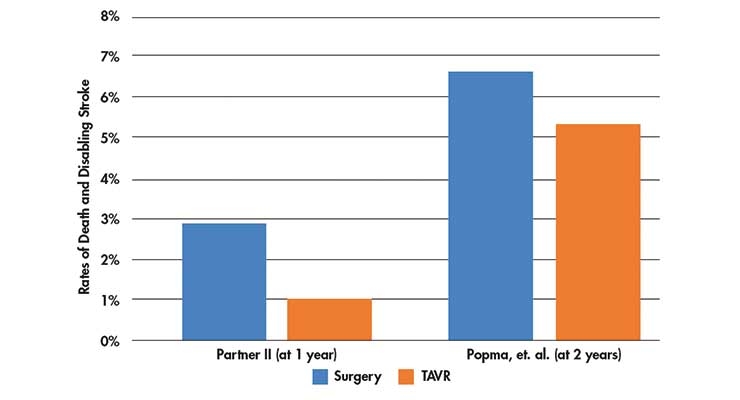
Table 1: Death and disabling stroke rates in the Partner II trial (at 1 year)2 and the Popma, et. al trial (at 2 years)1.
Both studies were randomized controlled studies that followed death, disabling stroke, and hospitalization at one- and two-years post-procedure. In the Partner II trial, the rates of death and disabling stroke were 2.9 percent with surgical aortic valve replacement performed via open heart surgery vs. 1 percent with the TAVR procedure. The Popma, et. al. study found death or disabling stroke rates of 6.7 percent with surgical aortic valve replacement vs. 5.3 percent with TAVR at two years.

Table 2: The difference in the cost of a TAVR procedure when compared to surgical aortic valve replacement.3
Further, a significant cost argument can be made for TAVR. In the Partner II trial, procedure cost was approximately $20,0003 higher with TAVR than surgical aortic valve replacement performed via open heart surgery. The total cost difference between surgical aortic valve replacement and TAVR, however, show costs were just $2,888 more with XT-TAVR (P=0.014) and $4,155 lower with S3-TAVR (P<0.001). This was due to a reduction in length of stay with TAVR. In addition, follow-up costs were significantly lower with TAVR (between $9,304 and $11,377; P<0.001) than with surgical aortic valve replacement (Table 2). Over the lifetime of the patient, TAVR is projected to lower the total cost of care by an average of $9,000.
Full disclosure—the trials were sponsored by Edwards Lifesciences and Medtronic plc, the only medical device manufacturers who have obtained regulatory approval or clearance of TAVR valves.
With traditional surgical aortic valve replacement, the cardiac surgeon removes the diseased valve and sutures in a replacement. The diseased valve is completely removed from the heart. In comparison, Edwards’ valve is compacted onto a balloon catheter. When it reaches the aorta, the physician inflates the balloon to expand the TAVR valve to replace the failing valve of the patient. Medtronic’s TAVR valve, on the other hand, is nitinol based. After transfemoral access, the physician pulls back the sheath, releasing the new valve into place in the aorta.
Surgical aortic valve replacements have been performed for decades, and in multiple previous studies in intermediate-risk TAVR patients, data demonstrated that complication rates are equivalent or better for TAVR than for intermediate-risk surgical aortic valve replacement patients.4 Cardiac surgeons recognize valves placed during aortic valve replacement procedures will last at least 10 to 15 years. The next step for clinical studies will be to determine if TAVR valves have equivalent or longer lasting survival rates.
Why This Is Important
These trial results are a shot in the arm for minimally invasive transcatheter valve procedures, and a shot over the bow for open-heart surgery. Complications have proven to be lower with TAVR for stroke and death compared to surgical aortic valve replacement, and interventional cardiologists believe5 it will transform the standard of care for the majority of patients with deteriorating aortic valves.
FDA is expected to approve the procedure for patients judged to be low-risk. This means up to 20,000 more patients each year could meet the new guidelines for TAVR. Currently, approximately 60,000 patients diagnosed as intermediate- and high-risk can get the operation (Table 3).

Table 3: Future eligibility for TAVR and the effect on procedure rates.
We all know what happens in open-heart surgical procedures like surgical aortic valve replacement. The patient’s chest is cracked, and the heart is stopped to insert a replacement aortic valve. Recovery can take several months, and complication rates are high. In a TAVR procedure, transfemoral access is gained through the groin, the TAVR catheter is introduced, and it is threaded up to the heart—similar to the method to place a stent. The procedure is performed under conscious sedation, and like any interventional procedure without complications, the patient goes home in a few days.
These results would seem to shift the treatment paradigm toward more patients being prescribed for TAVR. Heart surgeons agree. “If I were a patient, I would choose TAVR,” said Dr. Gilbert Tang, a heart surgeon at the Icahn School of Medicine at Mount Sinai in New York, N.Y., who was not involved in the research.5
Failure of the aortic valve starts in patients with a hardening of the valve controlling blood flow from the heart to the body. The primary risk factor is age, and there is no cure other than to replace the valve.
The Medi-Vantage Perspective
Cardiac surgery of any form is a highly profitable money maker for hospitals; as such, hospital administrators are faced with a dilemma. Should they market TAVR to patients much in the same way as the Intuitive Surgical robotics system has been successfully marketed, or take the financial downside when lower-risk patients start demanding the TAVR procedure? TAVR valves cost more than surgical aortic valve replacement, and insurers are reported to pay the same amount for both procedures,5 so the lonely loser here is the hospital. Edwards Lifesciences and Medtronic aren’t taking that risk and are providing TAVR physician finders on their respective websites. Edwards and Medtronic are enjoying their position as first to market but, in the future, many more heart valves will be coming, and aortic valve prices will drop (by how much is anyone’s guess). In the coming years, medical device price strategy and commercialization strategy research will make or break fast-following aortic valve medical devices.
References
Maria Shepherd has more than 20 years of leadership experience in medical device/life science marketing in small startups and top-tier companies. After her industry career, including her role as vice president of marketing for Oridion Medical, where she boosted the company valuation prior to its acquisition by Covidien/Medtronic, director of marketing for Philips Medical, and senior management roles at Boston Scientific Corp., she founded Medi-Vantage. Medi-Vantage provides marketing, business strategy, and innovation research for the medical device, diagnostic, and digital health industries. The firm quantitatively and qualitatively sizes and segments opportunities, evaluates new technologies, provides marketing services, and assesses prospective acquisitions. Shepherd has taught marketing and product development courses and is a member of the Aligo Medtech Investment Committee (www.aligo.com). She can be reached at 855-343-3100. Visit her website at www.medi-vantage.com.

Table 1: Death and disabling stroke rates in the Partner II trial (at 1 year)2 and the Popma, et. al trial (at 2 years)1.
Both studies were randomized controlled studies that followed death, disabling stroke, and hospitalization at one- and two-years post-procedure. In the Partner II trial, the rates of death and disabling stroke were 2.9 percent with surgical aortic valve replacement performed via open heart surgery vs. 1 percent with the TAVR procedure. The Popma, et. al. study found death or disabling stroke rates of 6.7 percent with surgical aortic valve replacement vs. 5.3 percent with TAVR at two years.

Table 2: The difference in the cost of a TAVR procedure when compared to surgical aortic valve replacement.3
Further, a significant cost argument can be made for TAVR. In the Partner II trial, procedure cost was approximately $20,0003 higher with TAVR than surgical aortic valve replacement performed via open heart surgery. The total cost difference between surgical aortic valve replacement and TAVR, however, show costs were just $2,888 more with XT-TAVR (P=0.014) and $4,155 lower with S3-TAVR (P<0.001). This was due to a reduction in length of stay with TAVR. In addition, follow-up costs were significantly lower with TAVR (between $9,304 and $11,377; P<0.001) than with surgical aortic valve replacement (Table 2). Over the lifetime of the patient, TAVR is projected to lower the total cost of care by an average of $9,000.
Full disclosure—the trials were sponsored by Edwards Lifesciences and Medtronic plc, the only medical device manufacturers who have obtained regulatory approval or clearance of TAVR valves.
With traditional surgical aortic valve replacement, the cardiac surgeon removes the diseased valve and sutures in a replacement. The diseased valve is completely removed from the heart. In comparison, Edwards’ valve is compacted onto a balloon catheter. When it reaches the aorta, the physician inflates the balloon to expand the TAVR valve to replace the failing valve of the patient. Medtronic’s TAVR valve, on the other hand, is nitinol based. After transfemoral access, the physician pulls back the sheath, releasing the new valve into place in the aorta.
Surgical aortic valve replacements have been performed for decades, and in multiple previous studies in intermediate-risk TAVR patients, data demonstrated that complication rates are equivalent or better for TAVR than for intermediate-risk surgical aortic valve replacement patients.4 Cardiac surgeons recognize valves placed during aortic valve replacement procedures will last at least 10 to 15 years. The next step for clinical studies will be to determine if TAVR valves have equivalent or longer lasting survival rates.
Why This Is Important
These trial results are a shot in the arm for minimally invasive transcatheter valve procedures, and a shot over the bow for open-heart surgery. Complications have proven to be lower with TAVR for stroke and death compared to surgical aortic valve replacement, and interventional cardiologists believe5 it will transform the standard of care for the majority of patients with deteriorating aortic valves.
FDA is expected to approve the procedure for patients judged to be low-risk. This means up to 20,000 more patients each year could meet the new guidelines for TAVR. Currently, approximately 60,000 patients diagnosed as intermediate- and high-risk can get the operation (Table 3).

Table 3: Future eligibility for TAVR and the effect on procedure rates.
We all know what happens in open-heart surgical procedures like surgical aortic valve replacement. The patient’s chest is cracked, and the heart is stopped to insert a replacement aortic valve. Recovery can take several months, and complication rates are high. In a TAVR procedure, transfemoral access is gained through the groin, the TAVR catheter is introduced, and it is threaded up to the heart—similar to the method to place a stent. The procedure is performed under conscious sedation, and like any interventional procedure without complications, the patient goes home in a few days.
These results would seem to shift the treatment paradigm toward more patients being prescribed for TAVR. Heart surgeons agree. “If I were a patient, I would choose TAVR,” said Dr. Gilbert Tang, a heart surgeon at the Icahn School of Medicine at Mount Sinai in New York, N.Y., who was not involved in the research.5
Failure of the aortic valve starts in patients with a hardening of the valve controlling blood flow from the heart to the body. The primary risk factor is age, and there is no cure other than to replace the valve.
The Medi-Vantage Perspective
Cardiac surgery of any form is a highly profitable money maker for hospitals; as such, hospital administrators are faced with a dilemma. Should they market TAVR to patients much in the same way as the Intuitive Surgical robotics system has been successfully marketed, or take the financial downside when lower-risk patients start demanding the TAVR procedure? TAVR valves cost more than surgical aortic valve replacement, and insurers are reported to pay the same amount for both procedures,5 so the lonely loser here is the hospital. Edwards Lifesciences and Medtronic aren’t taking that risk and are providing TAVR physician finders on their respective websites. Edwards and Medtronic are enjoying their position as first to market but, in the future, many more heart valves will be coming, and aortic valve prices will drop (by how much is anyone’s guess). In the coming years, medical device price strategy and commercialization strategy research will make or break fast-following aortic valve medical devices.
References
- http://bit.ly/mpo190401
- http://bit.ly/mpo190402
- http://bit.ly/mpo190404
- http://bit.ly/mpo190405
- http://bit.ly/mpo190403
Maria Shepherd has more than 20 years of leadership experience in medical device/life science marketing in small startups and top-tier companies. After her industry career, including her role as vice president of marketing for Oridion Medical, where she boosted the company valuation prior to its acquisition by Covidien/Medtronic, director of marketing for Philips Medical, and senior management roles at Boston Scientific Corp., she founded Medi-Vantage. Medi-Vantage provides marketing, business strategy, and innovation research for the medical device, diagnostic, and digital health industries. The firm quantitatively and qualitatively sizes and segments opportunities, evaluates new technologies, provides marketing services, and assesses prospective acquisitions. Shepherd has taught marketing and product development courses and is a member of the Aligo Medtech Investment Committee (www.aligo.com). She can be reached at 855-343-3100. Visit her website at www.medi-vantage.com.