Business Wire03.11.19
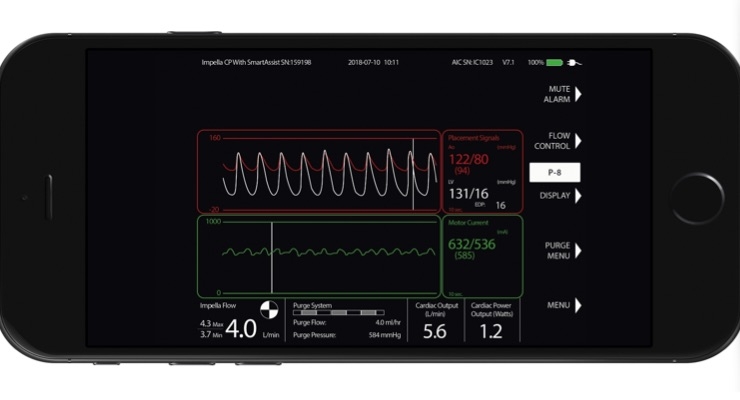
Abiomed has achieved CE Mark for Impella Connect, the first-of-its kind cloud-based technology that enables secure, real-time, remote viewing of the Impella console for physicians and hospital staff from anywhere with Internet connectivity. European CE Mark adds to Impella Connect’s previous U.S. Food and Drug Administration (FDA) pre-market approval.
Impella Connect uses real-time intelligence to help improve patient outcomes. In addition to allowing medical professionals to view their hospital’s consoles remotely, Impella Connect allows highly-trained staff at Abiomed’s 24x7 Clinical Support Center to provide medical professionals with expert evaluation of Impella data and real-time collaborative patient management.
“Impella Connect is an extremely valuable resource that allows me, as well as allied health professionals and nursing staff, to have direct visualization of data from the Impella console and to closely monitor patients on hemodynamic support, in real time,” said Rajeev L. Narayan, M.D., assistant director of Structural Heart Intervention and Director Interventional Mechanical Circulatory Support, Vassar Brothers Medical Center.
Based on the previous FDA PMA approval, Impella Connect, which is fully HIPAA compliant, is in a limited market release in the United States. Thirty-six hospital sites are currently using the technology on a regular basis to provide enhanced real-time support for their patients. Abiomed is launching Impella Connect in Europe through a controlled roll-out at hospital sites with established heart recovery protocols. The first hospital is University Heart Center in Hamburg, Germany.
“Impella Connect is a technological advancement which represents the next frontier of heart recovery products,” said Michael R. Minogue, president, chairman and CEO of Abiomed. “Impella Connect, along with our 24x7 onsite and on-call support, enables physicians, nurses and ICU staff to increase productivity, improve patient outcomes, and help patients return home with their native heart.”
The Impella 2.5 and Impella CP devices are FDA-approved to treat certain advanced heart failure patients undergoing elective and urgent percutaneous coronary interventions (PCI) such as stenting or balloon angioplasty, to re-open blocked coronary arteries. The Impella 2.5, Impella CP, Impella CP with SmartAssist, Impella 5.0 and Impella LD are FDA approved heart pumps used to treat heart attack or cardiomyopathy patients in cardiogenic shock, and have the unique ability to enable native heart recovery, allowing patients to return home with their own heart.
Based in Danvers, Mass., Abiomed Inc. provides medical devices that provide circulatory support. Its products are designed to enable the heart to rest by improving blood flow and/or performing the pumping of the heart.
Impella Connect uses real-time intelligence to help improve patient outcomes. In addition to allowing medical professionals to view their hospital’s consoles remotely, Impella Connect allows highly-trained staff at Abiomed’s 24x7 Clinical Support Center to provide medical professionals with expert evaluation of Impella data and real-time collaborative patient management.
“Impella Connect is an extremely valuable resource that allows me, as well as allied health professionals and nursing staff, to have direct visualization of data from the Impella console and to closely monitor patients on hemodynamic support, in real time,” said Rajeev L. Narayan, M.D., assistant director of Structural Heart Intervention and Director Interventional Mechanical Circulatory Support, Vassar Brothers Medical Center.
Based on the previous FDA PMA approval, Impella Connect, which is fully HIPAA compliant, is in a limited market release in the United States. Thirty-six hospital sites are currently using the technology on a regular basis to provide enhanced real-time support for their patients. Abiomed is launching Impella Connect in Europe through a controlled roll-out at hospital sites with established heart recovery protocols. The first hospital is University Heart Center in Hamburg, Germany.
“Impella Connect is a technological advancement which represents the next frontier of heart recovery products,” said Michael R. Minogue, president, chairman and CEO of Abiomed. “Impella Connect, along with our 24x7 onsite and on-call support, enables physicians, nurses and ICU staff to increase productivity, improve patient outcomes, and help patients return home with their native heart.”
The Impella 2.5 and Impella CP devices are FDA-approved to treat certain advanced heart failure patients undergoing elective and urgent percutaneous coronary interventions (PCI) such as stenting or balloon angioplasty, to re-open blocked coronary arteries. The Impella 2.5, Impella CP, Impella CP with SmartAssist, Impella 5.0 and Impella LD are FDA approved heart pumps used to treat heart attack or cardiomyopathy patients in cardiogenic shock, and have the unique ability to enable native heart recovery, allowing patients to return home with their own heart.
Based in Danvers, Mass., Abiomed Inc. provides medical devices that provide circulatory support. Its products are designed to enable the heart to rest by improving blood flow and/or performing the pumping of the heart.