Michael Barbella, Managing Editor04.04.19
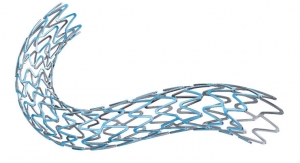
Cardiac stents have evolved tremendously since their birth in the late 1970s, having saved and improved the lives of countless heart attack and stroke patients. These devices also help boost kidney function, enhance lower-limb blood flow, and mitigate necessary leg amputations from gangrene.
Despite their many benefits, however, stents nonetheless maintain a major Achilles Heel: restenosis—i.e., recurring arterial narrowing after corrective surgery. The problem has improved significantly over the last several decades but still affects 5-7 percent of peripheral artery disease convalescents.
Technology is largely responsible for the drop in restenosis rates thanks to advancements in bare metal and drug-eluting stents (DES). The latter devices were developed to overcome the limitations of their metal predecessors, namely neointimal hyperplasia, repeat revascularization, and late stent thrombosis. The latest DES are designed to produce solid long-term clinical results and minimize both restenosis and stent thrombosis rates.
MPO’s March feature story “Free Flow” provides an in-depth look at the latest cardiac stent technologies and the future prospects for this burgeoning market. Dr. Alexander Uhl, senior vice president of Corporate Marketing at Biotronik, was among the experts interviewed for the feature; his full input is provided in the following Q&A.
Michael Barbella: Why did Biotronik design a covered coronary stent system (the first approved in 17 years)? What challenges were involved in developing the stent, and what kinds of market advantages does it provide for Biotronik?
Dr. Alexander Uhl: Biotronik continues to invest in bringing a full range of innovative coronary vascular intervention (CVI) products to the U.S. market, and the PK Papyrus is a unique, life-saving technology that is an important part of this portfolio. We invested in the development of PK Papyrus to meet a critical need in interventional cardiology. While coronary artery perforation is very uncommon—less than 4,000 per year in the United States—physicians must be fully prepared for this emergency event in order to save lives. When a perforation occurs during a percutaneous coronary intervention (PCI) procedure, physicians need a reliable covered stent that can be deployed quickly. Designed to deliver when seconds count, PK Papyrus is engineered on a single-stent, ultrathin strut platform that makes it more flexible and gives it a low crossing profile. Because of these features, PK Papyrus delivers more like a conventional stent with 5 French compatibility. We've improved every aspect of previous covered stents, transforming what was once a dreaded emergency use device into a reliable solution that physicians can deploy with confidence in critical situations.
PK Papyrus was approved for distribution by the FDA in September 2018 and has been trusted in international markets since receiving CE Marking in 2013. We made the decision to invest in PK Papyrus because patient care is paramount and Biotronik is committed to helping physicians and hospitals save lives and improve patient outcomes.
Barbella: What trends are impacting the coronary stent market? What factor(s) are driving these trends?
Dr. Uhl: As a private, independent company, Biotronik answers to values that dictate excellence and reliability before short-term gains. In the U.S. market, hospitals and health systems continue to leverage bargaining power and negotiate with vendors, driving consolidation amongst industry. The interventional cardiology market is also specifically affected by the growing focus on value-based care and long-term outcomes, which has brought changes in reimbursement policies, more intentional selection of PCI candidates, and various institutional cost-containment measures. Regardless of the market landscape, Biotronik focuses on proven, safe, and reliable solutions that demonstrate our commitment to care and foster relationships built on trust. We remain private by choice and are committed to partnering with hospitals to address the mounting cost pressures they're facing, which demand uncompromised quality.
Drug-eluting stents (DES) are used in more than 95 percent of all PCI procedures, with the occasional use of bare-metal stents (BMS) like Biotronik's PRO-Kinetic Energy. Because of the excellent clinical outcomes many modern DES have achieved, there has been commoditization in this space in the past decade that has made cost a primary driver for health system decision-makers. However, the BIOFLOW-V pivotal trial shows Biotronik's ultrathin Orsiro DES has demonstrated sustained improvement over Xience at multiple clinical endpoints. Orsiro's unprecedented outcomes may shift the market back toward a greater focus on clinical data.
Barbella: What factors are currently driving growth in the coronary vascular intervention market? How have these growth factors evolved over the past five years?
Dr. Uhl: Biotronik has been a player in the global vascular intervention space since 1994. In early 2017, we introduced our CVI portfolio to the United States with the launch of the PRO-Kinetic Energy and followed that with the approval of PK Papyrus in 2018. This market has remained largely stable over the past five years, with physicians performing approximately 950,000 PCI procedures annually. Future growth could be driven by an aging population and correlations to general lifestyle factors that may lead to coronary artery disease.
In alignment with value-based care, the decision-making process in many medtech sectors has evolved from physician preference or lowest cost to a discussion of positive patient outcomes and quality of care measures. Though PCI procedure volume is steady, the commoditization of the CVI market due to near-equivalent outcomes data has been eroding the average selling price (ASP) by approximately 10 percent year over year, causing a decline in total market value.
The U.S. DES market has suffered for too long from a lack of innovation. Patients deserve better outcomes, physicians deserve better performance, and healthcare administrators deserve more options. With Biotronik leading the way through meaningful innovations such as ultrathin struts and improved biocompatibility, the CVI market may refocus on clinical outcomes as the primary driving factor.
Barbella: What challenges are involved in developing a coronary stent? How has Biotronik overcome these challenges?
Dr. Uhl: Biotronik is a well-respected leader in CVI solutions outside the United States, where the value of our global portfolio has been recognized by the interventional cardiology community around the world. Our solutions have been proven in international markets and it made sense to invest in bringing these proven solutions to the United States as well. This is a market that rewards innovation and we are one of the most innovative companies the enter the CVI space in a long time. Stent research, design, manufacturing, and the clinical studies required to bring them to the U.S. market require decades of dedication and millions of dollars in capital investment. Biotronik has developed this technology for 25 years and optimized metallic stent design with the engineering of our ultrathin strut, cobalt chromium, double helix—the foundation on which we build our bare-metal, drug-eluting, and covered stents. We continue to invest in new technologies designed to help physicians improve patient care and ensure better outcomes. PK Papyrus and Orsiro are examples of Biotronik's continued investment.
Barbella: How is value-based care, new technologies like artificial intelligence, and material innovations affecting stent product designs?
Dr. Uhl: Value-based care is the natural extension of proven solutions with strong clinical outcomes. At Biotronik, we never rush to market. We research, innovate, and develop, then significantly invest in clinical studies to prove the capabilities of our products. Physicians trust Biotronik and know that our solutions will deliver reliable outcomes. In today's healthcare environment, that is the foundation of creating value. Clinical evidence has demostrated that Orsiro improves outcomes in comparison to Xience and Resolute; outcomes data is what really matters when creating and embracing value-based care.
Biotronik is the only company to manufacture a magnesium-based scaffold technology—the Magmaris Resorbable Magnesium Scaffold—which is available in CE and other international markets today. This materials innovation enables Magmaris to deliver and expand more like a conventional DES. Outcomes data from the BIOSOLVE-II and BIOSOLVE-III clinical studies show zero percent scaffold thrombosis at two years. These studies are investigating the safety and efficacy of Biotronik's Magmaris resorbable magnesium scaffold in Europe, South America, and Asia. Magmaris is not currently available in the United States.
Barbella: What new stent technologies are up and coming/on the horizon? What new stent technologies can we expect to see in the next several years?
Dr. Uhl: Resorbable scaffolds and polymer-free DES are currently available in CE markets. As investigators continue to conduct clinical trials and report their results, we may see those technologies enter the U.S. market as well.
Despite their many benefits, however, stents nonetheless maintain a major Achilles Heel: restenosis—i.e., recurring arterial narrowing after corrective surgery. The problem has improved significantly over the last several decades but still affects 5-7 percent of peripheral artery disease convalescents.
Technology is largely responsible for the drop in restenosis rates thanks to advancements in bare metal and drug-eluting stents (DES). The latter devices were developed to overcome the limitations of their metal predecessors, namely neointimal hyperplasia, repeat revascularization, and late stent thrombosis. The latest DES are designed to produce solid long-term clinical results and minimize both restenosis and stent thrombosis rates.
MPO’s March feature story “Free Flow” provides an in-depth look at the latest cardiac stent technologies and the future prospects for this burgeoning market. Dr. Alexander Uhl, senior vice president of Corporate Marketing at Biotronik, was among the experts interviewed for the feature; his full input is provided in the following Q&A.
Michael Barbella: Why did Biotronik design a covered coronary stent system (the first approved in 17 years)? What challenges were involved in developing the stent, and what kinds of market advantages does it provide for Biotronik?
Dr. Alexander Uhl: Biotronik continues to invest in bringing a full range of innovative coronary vascular intervention (CVI) products to the U.S. market, and the PK Papyrus is a unique, life-saving technology that is an important part of this portfolio. We invested in the development of PK Papyrus to meet a critical need in interventional cardiology. While coronary artery perforation is very uncommon—less than 4,000 per year in the United States—physicians must be fully prepared for this emergency event in order to save lives. When a perforation occurs during a percutaneous coronary intervention (PCI) procedure, physicians need a reliable covered stent that can be deployed quickly. Designed to deliver when seconds count, PK Papyrus is engineered on a single-stent, ultrathin strut platform that makes it more flexible and gives it a low crossing profile. Because of these features, PK Papyrus delivers more like a conventional stent with 5 French compatibility. We've improved every aspect of previous covered stents, transforming what was once a dreaded emergency use device into a reliable solution that physicians can deploy with confidence in critical situations.
PK Papyrus was approved for distribution by the FDA in September 2018 and has been trusted in international markets since receiving CE Marking in 2013. We made the decision to invest in PK Papyrus because patient care is paramount and Biotronik is committed to helping physicians and hospitals save lives and improve patient outcomes.
Barbella: What trends are impacting the coronary stent market? What factor(s) are driving these trends?
Dr. Uhl: As a private, independent company, Biotronik answers to values that dictate excellence and reliability before short-term gains. In the U.S. market, hospitals and health systems continue to leverage bargaining power and negotiate with vendors, driving consolidation amongst industry. The interventional cardiology market is also specifically affected by the growing focus on value-based care and long-term outcomes, which has brought changes in reimbursement policies, more intentional selection of PCI candidates, and various institutional cost-containment measures. Regardless of the market landscape, Biotronik focuses on proven, safe, and reliable solutions that demonstrate our commitment to care and foster relationships built on trust. We remain private by choice and are committed to partnering with hospitals to address the mounting cost pressures they're facing, which demand uncompromised quality.
Drug-eluting stents (DES) are used in more than 95 percent of all PCI procedures, with the occasional use of bare-metal stents (BMS) like Biotronik's PRO-Kinetic Energy. Because of the excellent clinical outcomes many modern DES have achieved, there has been commoditization in this space in the past decade that has made cost a primary driver for health system decision-makers. However, the BIOFLOW-V pivotal trial shows Biotronik's ultrathin Orsiro DES has demonstrated sustained improvement over Xience at multiple clinical endpoints. Orsiro's unprecedented outcomes may shift the market back toward a greater focus on clinical data.
Barbella: What factors are currently driving growth in the coronary vascular intervention market? How have these growth factors evolved over the past five years?
Dr. Uhl: Biotronik has been a player in the global vascular intervention space since 1994. In early 2017, we introduced our CVI portfolio to the United States with the launch of the PRO-Kinetic Energy and followed that with the approval of PK Papyrus in 2018. This market has remained largely stable over the past five years, with physicians performing approximately 950,000 PCI procedures annually. Future growth could be driven by an aging population and correlations to general lifestyle factors that may lead to coronary artery disease.
In alignment with value-based care, the decision-making process in many medtech sectors has evolved from physician preference or lowest cost to a discussion of positive patient outcomes and quality of care measures. Though PCI procedure volume is steady, the commoditization of the CVI market due to near-equivalent outcomes data has been eroding the average selling price (ASP) by approximately 10 percent year over year, causing a decline in total market value.
The U.S. DES market has suffered for too long from a lack of innovation. Patients deserve better outcomes, physicians deserve better performance, and healthcare administrators deserve more options. With Biotronik leading the way through meaningful innovations such as ultrathin struts and improved biocompatibility, the CVI market may refocus on clinical outcomes as the primary driving factor.
Barbella: What challenges are involved in developing a coronary stent? How has Biotronik overcome these challenges?
Dr. Uhl: Biotronik is a well-respected leader in CVI solutions outside the United States, where the value of our global portfolio has been recognized by the interventional cardiology community around the world. Our solutions have been proven in international markets and it made sense to invest in bringing these proven solutions to the United States as well. This is a market that rewards innovation and we are one of the most innovative companies the enter the CVI space in a long time. Stent research, design, manufacturing, and the clinical studies required to bring them to the U.S. market require decades of dedication and millions of dollars in capital investment. Biotronik has developed this technology for 25 years and optimized metallic stent design with the engineering of our ultrathin strut, cobalt chromium, double helix—the foundation on which we build our bare-metal, drug-eluting, and covered stents. We continue to invest in new technologies designed to help physicians improve patient care and ensure better outcomes. PK Papyrus and Orsiro are examples of Biotronik's continued investment.
Barbella: How is value-based care, new technologies like artificial intelligence, and material innovations affecting stent product designs?
Dr. Uhl: Value-based care is the natural extension of proven solutions with strong clinical outcomes. At Biotronik, we never rush to market. We research, innovate, and develop, then significantly invest in clinical studies to prove the capabilities of our products. Physicians trust Biotronik and know that our solutions will deliver reliable outcomes. In today's healthcare environment, that is the foundation of creating value. Clinical evidence has demostrated that Orsiro improves outcomes in comparison to Xience and Resolute; outcomes data is what really matters when creating and embracing value-based care.
Biotronik is the only company to manufacture a magnesium-based scaffold technology—the Magmaris Resorbable Magnesium Scaffold—which is available in CE and other international markets today. This materials innovation enables Magmaris to deliver and expand more like a conventional DES. Outcomes data from the BIOSOLVE-II and BIOSOLVE-III clinical studies show zero percent scaffold thrombosis at two years. These studies are investigating the safety and efficacy of Biotronik's Magmaris resorbable magnesium scaffold in Europe, South America, and Asia. Magmaris is not currently available in the United States.
Barbella: What new stent technologies are up and coming/on the horizon? What new stent technologies can we expect to see in the next several years?
Dr. Uhl: Resorbable scaffolds and polymer-free DES are currently available in CE markets. As investigators continue to conduct clinical trials and report their results, we may see those technologies enter the U.S. market as well.