PR Newswire02.20.19
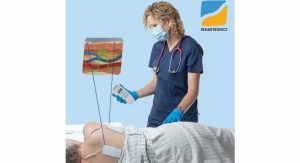
MagVenture, a medical device company providing drug-free depression treatment to the US since 2015, has added a new, FDA cleared robotic platform to their clinical system. This new solution, the TMS-Cobot, will help further elevate the rapidly growing field of neuromodulation, more commonly known as TMS—or Transcranial Magnetic Stimulation.
MagVenture TMS Therapy is an effective, non-invasive option for the large number of patients not responding to antidepressants. During treatment, magnetic pulses are applied to a particular area of the patient's brain with the use of a magnetic coil. This particular spot is the entry-point for the treatment of depression as it will reach the neural brain network controlling mood and emotion which may thus alleviate the depression. The patient is fully awake and may resume to his or her usual activities right after the TMS treatment session. The patient will typically receive 20-30 treatment sessions in total, one per weekday.
"While our customers express a very high level of satisfaction with the quality, cost-effectiveness, and longevity of the MagVenture TMS Therapy solution, some are also ready to take their TMS practice to the next level, so to speak, without compromising on quality or safety. Adding a robotic arm has two major advantages for these customers: Firstly, it provides a continuous head motion tracking system which actively follows the patient's possible head movement during treatment. Secondly, it will reduce the manual coil handling time for the TMS operator which especially is a key factor for clinics with a high number of patients," says Kerry Rome, MagVenture Inc. VP of Sales, before adding: "The TMS-Cobot is a good example of how the field of TMS is constantly evolving. We, as a TMS provider, need to stay tuned to meet customer and patient demands, for today and tomorrow."
The TMS-Cobot is developed by Axilum Robotics, a French company founded in 2011 by a team of leading experts in medical robotics. It is FDA indicated for the spatial positioning and orientation of the treatment coil of the MagVenture TMS Therapy system.
"We are very happy to partner with MagVenture Inc., who will distribute our device in the U.S. along with its MagVenture TMS Therapy system. We are convinced that this robotized system represents a major step forward for the implementation of TMS in the USA," said Michel Berg, CEO of Axilum Robotics.
MagVenture is a Danish medical device company specializing in non-invasive magnetic stimulation systems for the treatment of major depressive disorder and neuroscience research. In 2018, MagVenture was the first TMS company to receive FDA clearance for a much shorter protocol called Express TMS, which reduces the treatment time from the standard 37 minutes to just 3 minutes per session. The FDA clearance was based on the world's largest randomized controlled trial, also known as the THREE-D. The study found that 49 percent of the patients responded to the TMS treatment, and 32 percent achieved full remission (source: Blumberger et al, 2018, The Lancet).
MagVenture TMS Therapy is indicated for the treatment of Major Depressive Disorder in adult patients who have failed to receive satisfactory improvement from prior antidepressant medication in the current episode.
MagVenture TMS Therapy is an effective, non-invasive option for the large number of patients not responding to antidepressants. During treatment, magnetic pulses are applied to a particular area of the patient's brain with the use of a magnetic coil. This particular spot is the entry-point for the treatment of depression as it will reach the neural brain network controlling mood and emotion which may thus alleviate the depression. The patient is fully awake and may resume to his or her usual activities right after the TMS treatment session. The patient will typically receive 20-30 treatment sessions in total, one per weekday.
"While our customers express a very high level of satisfaction with the quality, cost-effectiveness, and longevity of the MagVenture TMS Therapy solution, some are also ready to take their TMS practice to the next level, so to speak, without compromising on quality or safety. Adding a robotic arm has two major advantages for these customers: Firstly, it provides a continuous head motion tracking system which actively follows the patient's possible head movement during treatment. Secondly, it will reduce the manual coil handling time for the TMS operator which especially is a key factor for clinics with a high number of patients," says Kerry Rome, MagVenture Inc. VP of Sales, before adding: "The TMS-Cobot is a good example of how the field of TMS is constantly evolving. We, as a TMS provider, need to stay tuned to meet customer and patient demands, for today and tomorrow."
The TMS-Cobot is developed by Axilum Robotics, a French company founded in 2011 by a team of leading experts in medical robotics. It is FDA indicated for the spatial positioning and orientation of the treatment coil of the MagVenture TMS Therapy system.
"We are very happy to partner with MagVenture Inc., who will distribute our device in the U.S. along with its MagVenture TMS Therapy system. We are convinced that this robotized system represents a major step forward for the implementation of TMS in the USA," said Michel Berg, CEO of Axilum Robotics.
MagVenture is a Danish medical device company specializing in non-invasive magnetic stimulation systems for the treatment of major depressive disorder and neuroscience research. In 2018, MagVenture was the first TMS company to receive FDA clearance for a much shorter protocol called Express TMS, which reduces the treatment time from the standard 37 minutes to just 3 minutes per session. The FDA clearance was based on the world's largest randomized controlled trial, also known as the THREE-D. The study found that 49 percent of the patients responded to the TMS treatment, and 32 percent achieved full remission (source: Blumberger et al, 2018, The Lancet).
MagVenture TMS Therapy is indicated for the treatment of Major Depressive Disorder in adult patients who have failed to receive satisfactory improvement from prior antidepressant medication in the current episode.