Business Wire04.05.19
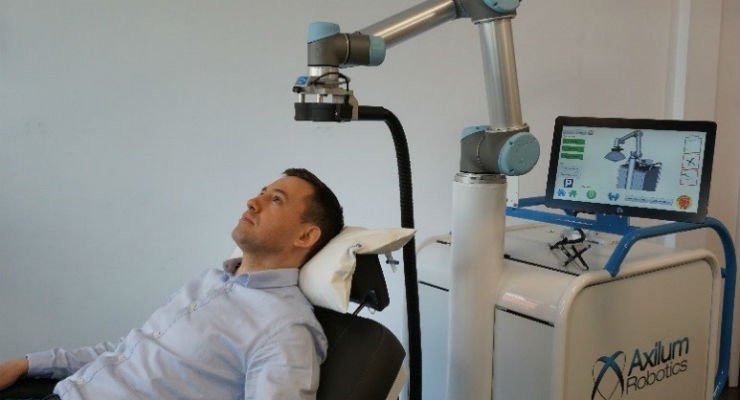
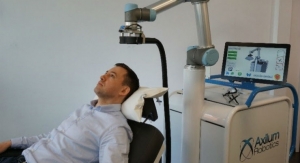
Axilum Robotics, specializing in the development of medical robots, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market the TMS-Cobot TS MV, indicated for the spatial positioning and orientation of the treatment coil of the MagVenture TMS Therapy system. The TMS-Cobot has already been CE Marked and approved for use in Europe.
After having successfully developed and launched outside of the United States (OUS) the TMS-Robot, the first robot designed to assist health care professionals in delivering Transcranial Magnetic Stimulation (TMS), based on an invention of ICube laboratory in Strasbourg, Axilum Robotics has reinforced its expertise in medical robotics with the development of a new platform based on collaborative robot technology.
TMS-Cobot, the first medical device built on this platform, allows the company to offer an affordable and versatile system, thanks particularly to a proprietary optical tracking system, which allows the control in real time of the position, orientation and contact of the stimulation device, with patient head motion compensation.
The compatible MagVenture TMS Therapy System is indicated in the United States for the treatment of major depressive disorder in adult patients who have failed to receive satisfactory improvement from prior antidepressant medication in the current episode. It was FDA cleared for this use on July 31, 2015.
Axilum Robotics’ TMS-Cobot TS MV will be distributed in the United States by MagVenture Inc. which is also the distributor of the MagVenture TMS Therapy System.
Transcranial Magnetic Stimulation is a rapidly expanding, non-invasive neurostimulation technique, particularly in the United States, where TMS has been covered by health insurance since 2012 and there is an increasing awareness of its current limitations when the coil is held manually or attached to a passive holder, requiring the patient to stay perfectly still.
“TMS-Cobot will allow us to better address the needs of the TMS therapeutic market by providing an affordable solution to allow precise TMS delivery while relieving the operator from a demanding and time-consuming task and reducing the movement constraints on the patient. It is important for the user to maximize the chance to deliver the stimulation dose at the right location and our medical robots help make this possible!” explained Michel Berg, CEO of Axilum Robotics. “We are very happy to partner with MagVenture Inc. who will distribute our device in the U.S. with its MagV TMS Therapy System. We are convinced that this robotized system represents a major step forward for the implementation of TMS in the U.S."
Axilum Robotics was founded in 2011 in Strasbourg, France, by a team of experts in medical robotics. The objective of the company is to provide researchers and health care professionals with robotic solutions to improve both technical medical procedures and medical resources management. In 2013, the company launched the TMS-Robot OUS, the first CE marked medical robot specifically designed for Transcranial Magnetic Stimulation.
TMS is a non-invasive neurostimulation technique. Its approved and potential applications are numerous, ranging from neuroscience research to the treatment of drug resistant neurological or psychiatric diseases. The procedure is usually implemented manually or with a fixed stand supporting the coil. Axilum Robotics has been ISO 13485 certified for its Quality Management System since 2013.
Beyond its activities in Transcranial Magnetic Stimulation, Axilum Robotics is member of a consortium engaged in the development of a device intended for blood brain barrier opening using ultrasound (3BOPUS, supported by National Research Agency) and member of a consortium for the development of a device for the treatment of bone metastases using ultrasound (UFOGUIDE, supported by French Unique Interministerial Fund).
After having successfully developed and launched outside of the United States (OUS) the TMS-Robot, the first robot designed to assist health care professionals in delivering Transcranial Magnetic Stimulation (TMS), based on an invention of ICube laboratory in Strasbourg, Axilum Robotics has reinforced its expertise in medical robotics with the development of a new platform based on collaborative robot technology.
TMS-Cobot, the first medical device built on this platform, allows the company to offer an affordable and versatile system, thanks particularly to a proprietary optical tracking system, which allows the control in real time of the position, orientation and contact of the stimulation device, with patient head motion compensation.
The compatible MagVenture TMS Therapy System is indicated in the United States for the treatment of major depressive disorder in adult patients who have failed to receive satisfactory improvement from prior antidepressant medication in the current episode. It was FDA cleared for this use on July 31, 2015.
Axilum Robotics’ TMS-Cobot TS MV will be distributed in the United States by MagVenture Inc. which is also the distributor of the MagVenture TMS Therapy System.
Transcranial Magnetic Stimulation is a rapidly expanding, non-invasive neurostimulation technique, particularly in the United States, where TMS has been covered by health insurance since 2012 and there is an increasing awareness of its current limitations when the coil is held manually or attached to a passive holder, requiring the patient to stay perfectly still.
“TMS-Cobot will allow us to better address the needs of the TMS therapeutic market by providing an affordable solution to allow precise TMS delivery while relieving the operator from a demanding and time-consuming task and reducing the movement constraints on the patient. It is important for the user to maximize the chance to deliver the stimulation dose at the right location and our medical robots help make this possible!” explained Michel Berg, CEO of Axilum Robotics. “We are very happy to partner with MagVenture Inc. who will distribute our device in the U.S. with its MagV TMS Therapy System. We are convinced that this robotized system represents a major step forward for the implementation of TMS in the U.S."
Axilum Robotics was founded in 2011 in Strasbourg, France, by a team of experts in medical robotics. The objective of the company is to provide researchers and health care professionals with robotic solutions to improve both technical medical procedures and medical resources management. In 2013, the company launched the TMS-Robot OUS, the first CE marked medical robot specifically designed for Transcranial Magnetic Stimulation.
TMS is a non-invasive neurostimulation technique. Its approved and potential applications are numerous, ranging from neuroscience research to the treatment of drug resistant neurological or psychiatric diseases. The procedure is usually implemented manually or with a fixed stand supporting the coil. Axilum Robotics has been ISO 13485 certified for its Quality Management System since 2013.
Beyond its activities in Transcranial Magnetic Stimulation, Axilum Robotics is member of a consortium engaged in the development of a device intended for blood brain barrier opening using ultrasound (3BOPUS, supported by National Research Agency) and member of a consortium for the development of a device for the treatment of bone metastases using ultrasound (UFOGUIDE, supported by French Unique Interministerial Fund).