Business Wire03.25.19
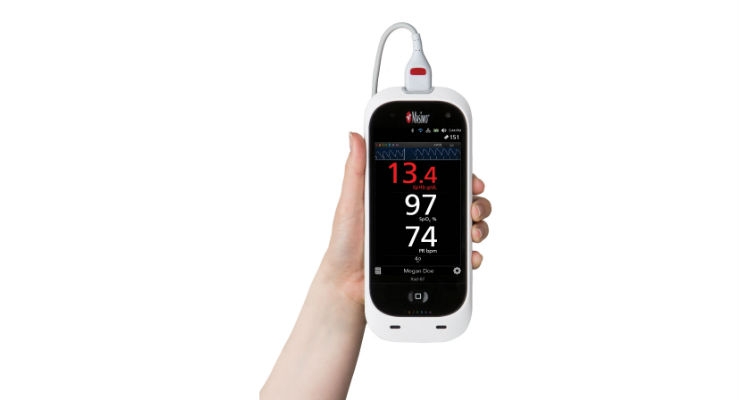
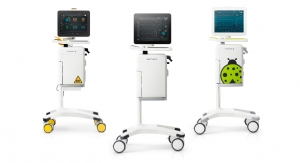
Masimo announced FDA clearance of the Rad-67 Pulse CO-Oximeter with Spot-check Next Generation SpHb monitoring technology and the rainbow DCI-mini Reusable Sensor. Rad-67 offers rainbow noninvasive hemoglobin measurement (SpHb) and Measure-through Motion and Low Perfusion SET pulse oximetry in a compact, portable spot-check monitoring device. When used with the rainbow DCI-mini sensor, Rad-67 provides spot-check monitoring with Next Generation SpHb.
Next Generation SpHb technology significantly advances the forefront of noninvasive portable hemoglobin spot-check monitoring: SpHb field performance is enhanced across all hemoglobin ranges through faster measurement results and improved repeatability.1
Rad-67’s ability to provide portable spot-check monitoring measurements of both oxygen saturation and noninvasive hemoglobin makes it a single-device solution in multiple clinical and non-clinical settings, such as emergency rooms, pre-/post-surgery settings, and physicians’ offices. Rad-67 features a rechargeable battery with six-hour run time, powering a high-resolution color display with intuitive touchscreen navigation that automatically adjusts brightness to optimize visibility in a variety of care settings. Rad-67’s feedback screens provide real-time user guidance during SpHb measurement to help reduce error, while the slim-profile sensor connector port is designed to provide tactile feedback upon proper connection.
Rad-67 provides convenient historical data review directly on the device, with unique patient identifiers to help improve organization of records and workflow. Built-in wireless connectivity will enable data transfer to the EMR and printing results at the point of care, which may help reduce the likelihood of transcription errors2 or incomplete case records.
Professor Andrew Klein, Chair of the Department of Anesthesia and Intensive Care at Royal Papworth Hospital in Cambridge, England, and Editor-in-Chief of Anaesthesia, commented, “The Rad-67 is incredibly easy to use and gives a quick result, perfect in our busy pre-operative clinic. We have found it particularly useful as it helps us target those patients who may need further testing.”
Joe Kiani, founder and CEO of Masimo, said, “We’re proud to launch Rad-67, Spot-check Next Generation SpHb monitoring, and the rainbow DCI-mini in the United States. Spot-check Next Generation SpHb monitoring represents a significant enhancement to the noninvasive measurement we invented a decade ago—a measurement we look forward to continuing to improve.”
Rad-67 spot-check SpHb monitoring is not cleared for use on pediatric patients, pregnant patients, and patients with renal disease. SpHb is not intended to replace laboratory blood testing. Blood samples should be analyzed by laboratory instruments prior to clinical decision making.
References
1 Masimo field data on file.
2 The Value of Medical Device Interoperability. West Health Institute. 2013.
Next Generation SpHb technology significantly advances the forefront of noninvasive portable hemoglobin spot-check monitoring: SpHb field performance is enhanced across all hemoglobin ranges through faster measurement results and improved repeatability.1
Rad-67’s ability to provide portable spot-check monitoring measurements of both oxygen saturation and noninvasive hemoglobin makes it a single-device solution in multiple clinical and non-clinical settings, such as emergency rooms, pre-/post-surgery settings, and physicians’ offices. Rad-67 features a rechargeable battery with six-hour run time, powering a high-resolution color display with intuitive touchscreen navigation that automatically adjusts brightness to optimize visibility in a variety of care settings. Rad-67’s feedback screens provide real-time user guidance during SpHb measurement to help reduce error, while the slim-profile sensor connector port is designed to provide tactile feedback upon proper connection.
Rad-67 provides convenient historical data review directly on the device, with unique patient identifiers to help improve organization of records and workflow. Built-in wireless connectivity will enable data transfer to the EMR and printing results at the point of care, which may help reduce the likelihood of transcription errors2 or incomplete case records.
Professor Andrew Klein, Chair of the Department of Anesthesia and Intensive Care at Royal Papworth Hospital in Cambridge, England, and Editor-in-Chief of Anaesthesia, commented, “The Rad-67 is incredibly easy to use and gives a quick result, perfect in our busy pre-operative clinic. We have found it particularly useful as it helps us target those patients who may need further testing.”
Joe Kiani, founder and CEO of Masimo, said, “We’re proud to launch Rad-67, Spot-check Next Generation SpHb monitoring, and the rainbow DCI-mini in the United States. Spot-check Next Generation SpHb monitoring represents a significant enhancement to the noninvasive measurement we invented a decade ago—a measurement we look forward to continuing to improve.”
Rad-67 spot-check SpHb monitoring is not cleared for use on pediatric patients, pregnant patients, and patients with renal disease. SpHb is not intended to replace laboratory blood testing. Blood samples should be analyzed by laboratory instruments prior to clinical decision making.
References
1 Masimo field data on file.
2 The Value of Medical Device Interoperability. West Health Institute. 2013.