Business Wire01.31.19
Senseonics Holdings Inc. a medical technology company focused on the development and commercialization of a long-term, implantable continuous glucose monitoring (CGM) system for people with diabetes, has announced that they received notice from FDA that the Eversense Sensor is no longer contraindicated for MRI scanning.
“Based on our testing, we have demonstrated that it is safe for patients to leave the Eversense Sensor in place, even when they need to have an MRI,” said Tim Goodnow, president and CEO of Senseonics. “Now patients using Eversense CGM do not need to worry about an emergency MRI or delay getting a scheduled MRI based on their glucose sensor. All other CGMs currently on the market are required to be removed before an MRI scan, according to their FDA indications. This is a first for the CGM category.”
A patient with this device can be safely scanned in an MR system meeting the following conditions:
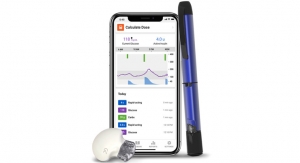
The Eversense CGM System consists of a fluorescence-based sensor, a smart transmitter worn over the sensor to facilitate data communication, and a mobile app for displaying glucose values, trends, and alerts. In addition to featuring the first long-term and first implantable CGM sensor, the system is also first to feature a smart transmitter that provides wearers with discreet on-body vibratory alerts for high and low glucose and that can be removed, recharged and re-adhered without discarding the sensor. The sensor is inserted subcutaneously in the upper arm by a health care provider via a brief in-office procedure. Now patients can safely get an MRI while still wearing the Eversense Sensor—the only CGM sensor that is indicated by the FDA for this use.
“Based on our testing, we have demonstrated that it is safe for patients to leave the Eversense Sensor in place, even when they need to have an MRI,” said Tim Goodnow, president and CEO of Senseonics. “Now patients using Eversense CGM do not need to worry about an emergency MRI or delay getting a scheduled MRI based on their glucose sensor. All other CGMs currently on the market are required to be removed before an MRI scan, according to their FDA indications. This is a first for the CGM category.”
A patient with this device can be safely scanned in an MR system meeting the following conditions:
- Static magnetic field of 1.5T or 3.0T
- Maximum spatial field gradient of 2000 gauss/cm (20 T/m)
- Maximum MR system reported, whole body averaged specific absorption rate (SAR) of 4 W/kg (First Level Controlled Operating Mode)
The Eversense CGM System consists of a fluorescence-based sensor, a smart transmitter worn over the sensor to facilitate data communication, and a mobile app for displaying glucose values, trends, and alerts. In addition to featuring the first long-term and first implantable CGM sensor, the system is also first to feature a smart transmitter that provides wearers with discreet on-body vibratory alerts for high and low glucose and that can be removed, recharged and re-adhered without discarding the sensor. The sensor is inserted subcutaneously in the upper arm by a health care provider via a brief in-office procedure. Now patients can safely get an MRI while still wearing the Eversense Sensor—the only CGM sensor that is indicated by the FDA for this use.