Abbott Laboratories08.02.21
The U.S. Food and Drug Administration (FDA) has cleared Abbott’s FreeStyle Libre 2 iOS application for use with compatible iPhones1, providing a comprehensive digital offering for its FreeStyle Libre 2 integrated continuous glucose monitoring (iCGM) system. Providing breakthrough technology that is accessible and affordable4, the FreeStyle Libre portfolio is the number one continuous glucose monitoring (CGM) system worldwide5 and most prescribed in the U.S.6, changing the lives of nearly 3.5 million people across more than 50 countries including 1 million users in the U.S.
Approved for adults and children (4 and older) with diabetes, the new FreeStyle Libre 2 app creates a seamless experience for users and healthcare professionals. The app enables users to get glucose readings directly on their iPhones without the use of a reader. A key benefit of the app is the ability for caregivers to remotely monitor their loved one's glucose readings and get real-time alarms via the LibreLinkUp app.7
"The demands of living with diabetes can be overwhelming, and there's a critical need to improve the way people with diabetes manage their condition—easily, affordably, accurately—and in a way that seamlessly fits into their everyday lives," said Jared Watkin, senior vice president, Diabetes Care, Abbott. "The FreeStyle Libre 2 iOS app streamlines how people manage their diabetes on their iPhones—empowering users with the information they need 24/7 while improving their health on the go."
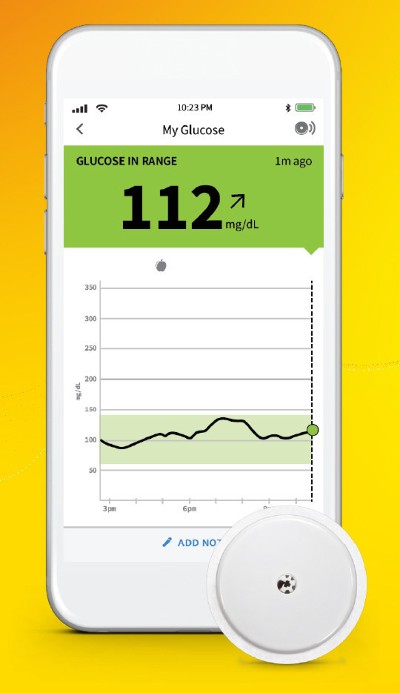
The app creates a comprehensive, digital experience for FreeStyle Libre users and healthcare professionals and is the only sensor-based glucose monitoring app that allows users to check their glucose with a compatible iPhone every minute with optional real-time alarms.
According to a recent Gallup poll, 1 in 5 Americans currently tracks health statistics using a mobile app.8 Free to download on the iOS App Store soon, the FreeStyle Libre 2 app will offer personalized, up-to-the-minute glucose data for people with diabetes who are using FreeStyle Libre 2 glucose sensors. By scanning the sensor with the FreeStyle Libre 2 app, users will get their current glucose reading and trend arrow which can help them determine how food, exercise and other lifestyle factors impact diabetes management.
With a 14-day wear time, FreeStyle Libre 2 is the longest-lasting iCGM sensor currently on the market.9 Available at pharmacies at a fraction of the cost of other CGM systems,10 the FreeStyle Libre 2 system is widely accessible to people with diabetes. Integrated features include:
"The pandemic taught us the importance of connected devices in managing chronic conditions remotely, and we continue to see the benefit from the latest advances in digital health," said Kurt Midyett, M.D., pediatric endocrinologist and medical director at Midwest Pediatric Specialists. "Continuous glucose monitoring is one of the most significant health tech innovations in the last decade, and I see the life-changing benefits first-hand from my patients every day. As a doctor, it's invaluable to receive my patients' glucose data remotely via systems like LibreView—enabling more meaningful conversations with my patients, and ultimately, improving treatment decisions and their long-term health."
Abbott has secured partial or full reimbursement for the FreeStyle Libre system in 38 countries, including Canada, France, Germany, Japan, the United Kingdom, and the U.S.
The FreeStyle Libre 2 iOS app will be available soon in the U.S. to download on the App Store.
References
1 The FreeStyle Libre 2 app is only compatible with certain mobile devices and operating systems. Please check our website for more information about device compatibility before using the app. Use of the FreeStyle Libre 2 app requires registration with LibreView.
2 The High Glucose alarm level can be set between 120-400 mg/dL and the Low Glucose alarm level can be set between 60 – 100 mg/dL Notifications will only be received when alarms are turned on and the sensor is within 20 feet of the reading device. Critical Alerts must be enabled to receive alarms and alerts on the smartphone.
3 The LibreLinkUp app is only compatible with certain mobile device and operating systems. Please check www.librelinkup.com for more information about device compatibility before using the app. Use of FreeStyle Libre-compatible apps and LibreLinkUp may require registration with LibreView. The LibreLinkUp mobile app is not intended to be a primary glucose monitor: users must consult their primary device(s) and consult a healthcare professional before making any medical interpretation and therapy adjustments from the information provided by the app.
4 Based on a comparison of list prices of the FreeStyle Libre portfolio versus competitor CGM systems. The actual cost to patients may or may not be lower than other CGM systems, depending on the amount covered by insurance, if any.
5 Data on file, Abbott Diabetes Care. Data based on the number of users worldwide for the FreeStyle Libre system compared to the number of users for other leading personal-use, sensor-based glucose monitoring systems
6 Data based on the number of patients assigned to each manufacturer based on last filled prescription in US Retail Pharmacy and DME.
7 The LibreLinkUp app is only compatible with certain mobile devices and operating systems. Please check www.LibreLinkUp.com for more information about device compatibility before using the app. Use of the LibreLinkUp app requires registration with LibreView.
8 Gallup News Service; Gallup Poll Social Series Health and Healthcare, Nov. 1 – 4, 2019
9 Data on file, Abbott Diabetes Care.
10 Based on a comparison of list prices of the FreeStyle Libre portfolio versus competitor CGM systems. The actual cost to patients may or may not be lower than other CGM systems, depending on the amount covered by insurance, if any.
11 Based on FDA iCGM special controls.
12 The LibreView data management software is intended for use by both patients and healthcare professionals to assist people with diabetes and their healthcare professionals in the review, analysis and evaluation of historical glucose meter data to support effective diabetes management. The LibreView software is not intended to provide treatment decisions or to be used as a substitute for professional healthcare advice.
13 Data on file, Abbott Diabetes Care. (Percent of PAT Accounts – percent of Patient LibreView Accounts that enabled remote monitoring)
Approved for adults and children (4 and older) with diabetes, the new FreeStyle Libre 2 app creates a seamless experience for users and healthcare professionals. The app enables users to get glucose readings directly on their iPhones without the use of a reader. A key benefit of the app is the ability for caregivers to remotely monitor their loved one's glucose readings and get real-time alarms via the LibreLinkUp app.7
"The demands of living with diabetes can be overwhelming, and there's a critical need to improve the way people with diabetes manage their condition—easily, affordably, accurately—and in a way that seamlessly fits into their everyday lives," said Jared Watkin, senior vice president, Diabetes Care, Abbott. "The FreeStyle Libre 2 iOS app streamlines how people manage their diabetes on their iPhones—empowering users with the information they need 24/7 while improving their health on the go."

The app creates a comprehensive, digital experience for FreeStyle Libre users and healthcare professionals and is the only sensor-based glucose monitoring app that allows users to check their glucose with a compatible iPhone every minute with optional real-time alarms.
With a 14-day wear time, FreeStyle Libre 2 is the longest-lasting iCGM sensor currently on the market.9 Available at pharmacies at a fraction of the cost of other CGM systems,10 the FreeStyle Libre 2 system is widely accessible to people with diabetes. Integrated features include:
- Optional real-time glucose alarms2 that automatically alert users when glucose is high or low
- Unsurpassed 14-day accuracy11 with readings every minute and an eight-hour glucose history, including comprehensive view of trends
- Seamless integration with Abbott's connected digital health tools—the LibreLinkUp app and LibreView12, a secure cloud-based data management platform—allowing users to easily share their glucose readings with healthcare professionals and caregivers. Accessible remotely, use of LibreView increased about 70 percent over the past year.13
"The pandemic taught us the importance of connected devices in managing chronic conditions remotely, and we continue to see the benefit from the latest advances in digital health," said Kurt Midyett, M.D., pediatric endocrinologist and medical director at Midwest Pediatric Specialists. "Continuous glucose monitoring is one of the most significant health tech innovations in the last decade, and I see the life-changing benefits first-hand from my patients every day. As a doctor, it's invaluable to receive my patients' glucose data remotely via systems like LibreView—enabling more meaningful conversations with my patients, and ultimately, improving treatment decisions and their long-term health."
Abbott has secured partial or full reimbursement for the FreeStyle Libre system in 38 countries, including Canada, France, Germany, Japan, the United Kingdom, and the U.S.
The FreeStyle Libre 2 iOS app will be available soon in the U.S. to download on the App Store.
References
1 The FreeStyle Libre 2 app is only compatible with certain mobile devices and operating systems. Please check our website for more information about device compatibility before using the app. Use of the FreeStyle Libre 2 app requires registration with LibreView.
2 The High Glucose alarm level can be set between 120-400 mg/dL and the Low Glucose alarm level can be set between 60 – 100 mg/dL Notifications will only be received when alarms are turned on and the sensor is within 20 feet of the reading device. Critical Alerts must be enabled to receive alarms and alerts on the smartphone.
3 The LibreLinkUp app is only compatible with certain mobile device and operating systems. Please check www.librelinkup.com for more information about device compatibility before using the app. Use of FreeStyle Libre-compatible apps and LibreLinkUp may require registration with LibreView. The LibreLinkUp mobile app is not intended to be a primary glucose monitor: users must consult their primary device(s) and consult a healthcare professional before making any medical interpretation and therapy adjustments from the information provided by the app.
4 Based on a comparison of list prices of the FreeStyle Libre portfolio versus competitor CGM systems. The actual cost to patients may or may not be lower than other CGM systems, depending on the amount covered by insurance, if any.
5 Data on file, Abbott Diabetes Care. Data based on the number of users worldwide for the FreeStyle Libre system compared to the number of users for other leading personal-use, sensor-based glucose monitoring systems
6 Data based on the number of patients assigned to each manufacturer based on last filled prescription in US Retail Pharmacy and DME.
7 The LibreLinkUp app is only compatible with certain mobile devices and operating systems. Please check www.LibreLinkUp.com for more information about device compatibility before using the app. Use of the LibreLinkUp app requires registration with LibreView.
8 Gallup News Service; Gallup Poll Social Series Health and Healthcare, Nov. 1 – 4, 2019
9 Data on file, Abbott Diabetes Care.
10 Based on a comparison of list prices of the FreeStyle Libre portfolio versus competitor CGM systems. The actual cost to patients may or may not be lower than other CGM systems, depending on the amount covered by insurance, if any.
11 Based on FDA iCGM special controls.
12 The LibreView data management software is intended for use by both patients and healthcare professionals to assist people with diabetes and their healthcare professionals in the review, analysis and evaluation of historical glucose meter data to support effective diabetes management. The LibreView software is not intended to provide treatment decisions or to be used as a substitute for professional healthcare advice.
13 Data on file, Abbott Diabetes Care. (Percent of PAT Accounts – percent of Patient LibreView Accounts that enabled remote monitoring)























