Michael Barbella, Managing Editor06.07.21
The world's diabetes care devices market is predicted to grow a solid 6.1 percent annually over the next six years to reach $39.4 billion by 2027 (up from $23.3 billion in 2018), according to a study from The Insight Partners.
BD; Novo Nordisk A/S; B. Braun Melsungen AG; Insulet Corporation; Medtronic plc; Tandem Diabetes Care Inc.; Eli Lilly and Company; Dexcom Inc.; Terumo Corporation; and F. Hoffman-LA Roche Ltd are among the key companies operating in the global diabetes care devices market. Leading players are focusing on the expansion and diversification of their market presence, and acquisition of new customer base, thereby tapping prevailing business opportunities.
In June 2018, Medtronic received an approval from the U.S. Food and Drug Administration for the MiniMed (TM) 670G system to cure the type 1 diabetes patients for the age group of 7–13 years. The system consists of the most advanced SmartGuard technology and Continuous Glucose Monitoring (CGM)—the Guardian Sensor 3 to power the delivery of insulin every five minutes according to the sensor glucose values.
In March 2019, Tandem Diabetes Care announced that it was registered as an approved vendor of insulin pumps and supplies under the Assistive Devices Program (ADP) in Ontario, Canada.
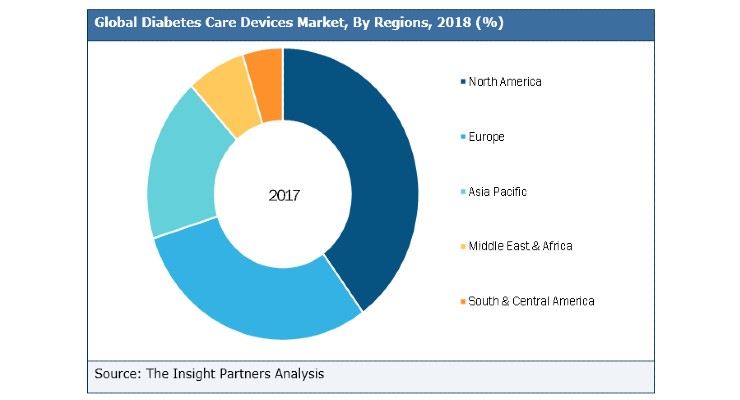
In 2019, North America dominated the global diabetes care devices market. The high cost of diabetes care devices and risks associated with the insulin delivery devices are hampering the market. Moreover, awareness programs and initiatives for preventing the rise in incidence of diabetes and change in the reimbursement scenarios are likely to add up to the growth during the forecast period.
Based on product, the diabetes care devices market is segmented into glucose monitoring devices and insulin delivery devices. In 2018, the glucose monitoring devices segment held the largest share of the market, by product. Moreover, the glucose monitoring devices segment is expected to grow at the fastest CAGR during the forecast period. The glucose monitoring devices or glucose meters are the medical devices used to determine the approximate levels or the concentration of glucose in the blood of the patients living with diabetes. The concentration of the glucose level can be measured by various means such as through testing strips, lancets, and others. Therefore, owing to the presence of the various market players that are offering various products and rise in technological advancements are likely to propel the growth of the market during the forecast period. Moreover, The COVID-19 pandemic has brought a new urgency to the need to assess the feasibility of CGM in the hospital to preserve personal protective equipment (PPE) and limit health care workers’ exposure. Achieving even standard glycemic control of 7.8–10.0 mmol/L (140–180 mg/dL) is now a challenge for many hospitals in both the ICU and non-ICU areas for those infected with COVID-19. Similarly, the diabetic patients are more prone to get infected with coronavirus. Thus, the rising COVID-19 cases, is likely to drive the diabetes care devices.
Advancements in the healthcare industry are pushing the market players for more research and developments related to insulin delivery devices. There are many methods to deliver insulin into the body such as needles, insulin pens, and insulin pumps. Insulin pumps are small, computerized diabetes management devices, connected with cannula under the skin that is used to deliver a slow continuous level of insulin. The program can be controlled by the individual depending on the requirement. The optimal diabetes management depends on the accurate glucose monitoring devices. The increasing technological advancements in glucose monitoring devices have resulted into smaller amount of required blood volumes with improved accuracy. The ability to transfer data between the blood glucose (BG) meter and insulin delivery devices has been also improved. The increasing advancements in blood glucose (BG) monitoring technology have resulted in improved accuracy, smaller required blood volumes, and the ability to transfer data between the BG meter and insulin delivery devices. For instance, in September 2016, Medtronic’s MiniMed 670G which is a hybrid closed looped system that provide appropriate basal insulin doses in people 14 years of age and older with type 1 diabetes. In addition, in July 2018, Abbott received the FDA approval for the FreeStyle Libre 14 day Flash Glucose Monitoring system that allows people with diabetes to wear the sensor up to 14 days with high accuracy.
In terms of type, the diabetes care devices market is segmented into glucose monitoring devices and insulin delivery devices. In 2018, the glucose monitoring devices segment held the largest share of the market, by product. Moreover, the glucose monitoring devices segment is expected to grow at the fastest CAGR during 2019–2027. The glucose monitoring devices segment is further divided into glucometers, lancets, testing strips, and other glucose monitoring devices. In 2018, the testing strips segment held the largest market share.
The glucose monitoring devices or glucose meters are the medical devices used to determine the approximate levels or the concentration of glucose in the blood of the patients living with diabetes. The concentration of the glucose level can be measured by various means such as through testing strips, lancets, and others.
Pen needles and syringes are the most commonly used devices for injecting insulin to the diabetic patients. Various manufacturers are coming up with innovative products to sustain in the highly fragmented global diabetes care market. Moreover, easy regulatory approvals in the Asian countries have resulted into mid-sized companies entering the market and competing with local and established players. For instance, in May 2018, Hindustan Syringes and Medical Devices (HMD), one of the leading manufacturers of disposable syringes, introduced “Dispovan” insulin pen needle. It is the first made-in-India disposable pen needle that is technologically efficient and affordable than the currently available products in the market. Manufacturers are also focusing on development of pen needles with precise lubrication to reduce the pain sensation.
ARKRAY USA Inc., one of the leaders in diabetes care products, introduced TechLITE pen needles in July 2016 that comes with thin-wall technology and pin-point sharpening to reduce the pain during a delivery procedure. For instance, in September 2017, BD, one of the top players in the pen needles market, introduced a new variant of BD-Ultra Fine micro pen needles that has a dimension of 6 mm X 32 mm. In addition, to reduce the injection site repetition for drug administration, montméd Inc. in April 2017 introduced a new a variant of SiteSmart colored pen needles to keep the injection site tissue healthy and promote better insulin adoption. Thus, the technological advancements, coupled with increasing influx of new products into the market, are expected to propel the growth of global diabetes care devices market over the forecast period.
BD; Novo Nordisk A/S; B. Braun Melsungen AG; Insulet Corporation; Medtronic plc; Tandem Diabetes Care Inc.; Eli Lilly and Company; Dexcom Inc.; Terumo Corporation; and F. Hoffman-LA Roche Ltd are among the key companies operating in the global diabetes care devices market. Leading players are focusing on the expansion and diversification of their market presence, and acquisition of new customer base, thereby tapping prevailing business opportunities.
In June 2018, Medtronic received an approval from the U.S. Food and Drug Administration for the MiniMed (TM) 670G system to cure the type 1 diabetes patients for the age group of 7–13 years. The system consists of the most advanced SmartGuard technology and Continuous Glucose Monitoring (CGM)—the Guardian Sensor 3 to power the delivery of insulin every five minutes according to the sensor glucose values.
In March 2019, Tandem Diabetes Care announced that it was registered as an approved vendor of insulin pumps and supplies under the Assistive Devices Program (ADP) in Ontario, Canada.
In 2019, North America dominated the global diabetes care devices market. The high cost of diabetes care devices and risks associated with the insulin delivery devices are hampering the market. Moreover, awareness programs and initiatives for preventing the rise in incidence of diabetes and change in the reimbursement scenarios are likely to add up to the growth during the forecast period.
Based on product, the diabetes care devices market is segmented into glucose monitoring devices and insulin delivery devices. In 2018, the glucose monitoring devices segment held the largest share of the market, by product. Moreover, the glucose monitoring devices segment is expected to grow at the fastest CAGR during the forecast period. The glucose monitoring devices or glucose meters are the medical devices used to determine the approximate levels or the concentration of glucose in the blood of the patients living with diabetes. The concentration of the glucose level can be measured by various means such as through testing strips, lancets, and others. Therefore, owing to the presence of the various market players that are offering various products and rise in technological advancements are likely to propel the growth of the market during the forecast period. Moreover, The COVID-19 pandemic has brought a new urgency to the need to assess the feasibility of CGM in the hospital to preserve personal protective equipment (PPE) and limit health care workers’ exposure. Achieving even standard glycemic control of 7.8–10.0 mmol/L (140–180 mg/dL) is now a challenge for many hospitals in both the ICU and non-ICU areas for those infected with COVID-19. Similarly, the diabetic patients are more prone to get infected with coronavirus. Thus, the rising COVID-19 cases, is likely to drive the diabetes care devices.
Advancements in the healthcare industry are pushing the market players for more research and developments related to insulin delivery devices. There are many methods to deliver insulin into the body such as needles, insulin pens, and insulin pumps. Insulin pumps are small, computerized diabetes management devices, connected with cannula under the skin that is used to deliver a slow continuous level of insulin. The program can be controlled by the individual depending on the requirement. The optimal diabetes management depends on the accurate glucose monitoring devices. The increasing technological advancements in glucose monitoring devices have resulted into smaller amount of required blood volumes with improved accuracy. The ability to transfer data between the blood glucose (BG) meter and insulin delivery devices has been also improved. The increasing advancements in blood glucose (BG) monitoring technology have resulted in improved accuracy, smaller required blood volumes, and the ability to transfer data between the BG meter and insulin delivery devices. For instance, in September 2016, Medtronic’s MiniMed 670G which is a hybrid closed looped system that provide appropriate basal insulin doses in people 14 years of age and older with type 1 diabetes. In addition, in July 2018, Abbott received the FDA approval for the FreeStyle Libre 14 day Flash Glucose Monitoring system that allows people with diabetes to wear the sensor up to 14 days with high accuracy.
In terms of type, the diabetes care devices market is segmented into glucose monitoring devices and insulin delivery devices. In 2018, the glucose monitoring devices segment held the largest share of the market, by product. Moreover, the glucose monitoring devices segment is expected to grow at the fastest CAGR during 2019–2027. The glucose monitoring devices segment is further divided into glucometers, lancets, testing strips, and other glucose monitoring devices. In 2018, the testing strips segment held the largest market share.
The glucose monitoring devices or glucose meters are the medical devices used to determine the approximate levels or the concentration of glucose in the blood of the patients living with diabetes. The concentration of the glucose level can be measured by various means such as through testing strips, lancets, and others.
Pen needles and syringes are the most commonly used devices for injecting insulin to the diabetic patients. Various manufacturers are coming up with innovative products to sustain in the highly fragmented global diabetes care market. Moreover, easy regulatory approvals in the Asian countries have resulted into mid-sized companies entering the market and competing with local and established players. For instance, in May 2018, Hindustan Syringes and Medical Devices (HMD), one of the leading manufacturers of disposable syringes, introduced “Dispovan” insulin pen needle. It is the first made-in-India disposable pen needle that is technologically efficient and affordable than the currently available products in the market. Manufacturers are also focusing on development of pen needles with precise lubrication to reduce the pain sensation.
ARKRAY USA Inc., one of the leaders in diabetes care products, introduced TechLITE pen needles in July 2016 that comes with thin-wall technology and pin-point sharpening to reduce the pain during a delivery procedure. For instance, in September 2017, BD, one of the top players in the pen needles market, introduced a new variant of BD-Ultra Fine micro pen needles that has a dimension of 6 mm X 32 mm. In addition, to reduce the injection site repetition for drug administration, montméd Inc. in April 2017 introduced a new a variant of SiteSmart colored pen needles to keep the injection site tissue healthy and promote better insulin adoption. Thus, the technological advancements, coupled with increasing influx of new products into the market, are expected to propel the growth of global diabetes care devices market over the forecast period.