Maria Shepherd, President and Founder, Medi-Vantage04.01.21
Continuous glucose monitors (CGMs) are one of the most formidable diabetes management tools to be developed in decades.
The change in technology is impressive. “Decades ago, we were still checking urine sugars,” Dr. Egils Bogdanovics, a Connecticut-based endocrinologist, said in a recent article.1 “In the early 1980s, fingerstick blood glucose monitors came out. That was a big deal—a revolution in diabetes.”
FDA cleared the first CGM in 1999, and CGM technology has rapidly evolved. The most up-to-date CGMs sent data via Bluetooth to smart phones through the cloud, allowing patients to read their blood glucose levels through an app in real-time.
That standard of care is rapidly changing. According to a recent report,2 the CGM market is experiencing significant change with new trends that are expected to shift management of Type 1 and Type 2 for people with diabetes. CGM technology is improving with several new closed-loop systems expected to launch in the next year. The prediction has been made that traditional blood glucose meters will become obsolete in the face of closed loop CGM, which will see rapid growth as patients switch out old meters for new technology.
Telehealth may also be a CGM market driver if it is used to treat patients with diabetes by the care team. Virtual visits may decline to <50 percent of office visits post-COVID but are expected to remain high if integrated data access becomes the norm in telehealth platforms.
New CGM tech will change the way patients with diabetes manage their care. Fingerstick glucose monitoring provided readings that were only a snapshot in time. New CGMs will change all that. “Initially, the main emphasis was to avoid hypoglycemia,” said Bogdanovics.1 This was a big risk for patients with Type 1 diabetes, that they might not be aware that their blood sugar levels had dropped to hazardous levels, leaving them little time to manage the problem.
The newest CGMs will simplify the treatment of patients with diabetes because they are automated, closed loop, and require no finger sticks or need for calibration. However, all contain a warning—CGM IFUs state if glucose alerts and readings do not match symptoms or meet their expectations, patients should use a traditional blood glucose meter to make diabetes management decisions. Otherwise, new CGMs allow patients to read their glucose numbers with selected types of smart phones or devices. Patients can get alerts based on customized settings and share their glucose data with designated family, friends, or clinicians.
Why This Is Important
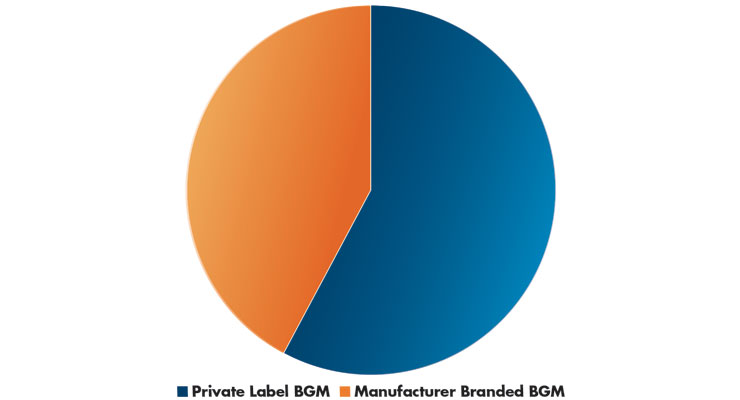
Market shares are changing based on the newest automated technologies, but in 2019, U.S. sales of private label blood glucose meters (BGM) were the largest segment, with manufacturer branded devices a distant second—$167.7 million vs. $122.4 million, respectively (Table 1).3 According to Statista, in 2019, the second best-selling blood glucose meter brand, after aggregated private label brands, was the Lifescan One Touch Ultra (formerly a Johnson & Johnson product but divested to Platinum Equity in 2018 for an estimated $2.1 billion4).
Walmart, CVS, and other market share holders will not give those sales up easily. These companies bring distribution power to the supplier of products for patients with diabetes, and emissaries are probably at the tables of all the manufacturers of CGM, like Medtronic, DexCom, and Insulet.
Diabetic Personal Care: A Big, Growing Market in the U.S.
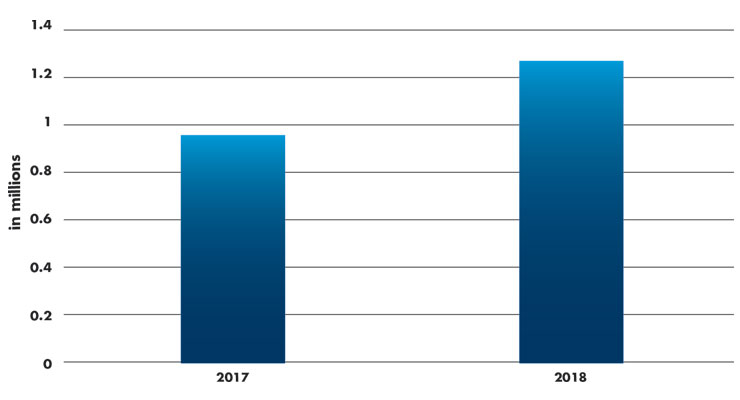
Diabetes has grown to global epidemic proportions and the market for diabetic personal care will continue to accelerate. It is reported that 1.26 million blood glucose meters were sold in the U.S. in 2018,2 an increase from reports of 960,000 blood glucose meters sold in 2017 (Table 2). That is a shocking increase of 31 percent.
According to Consumer Reports (CR), patients should choose a new CGM based on the features, benefits, and options that fit with their needs. CR also recommends patients compare the retail price of a new CGM to the cost of the continued use of blood glucose monitoring test strips with old BGM tech. Test strips were the true profit driver in this market and cost $18 to $184 per package of 100. CR estimates, depending upon patient diagnosis, the test strips can be an additional $265 to $2,685 or more per year for patients with diabetes. Replacement lances are an additional cost, and under some plans, test strips coverage depends upon the patient’s insulin requirements.
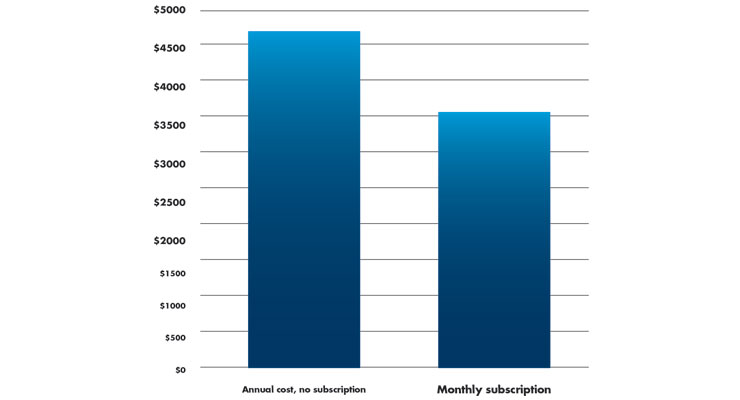
Another factor a patient should consider is insurance. Medicare covers some diabetes-related supplies. Some, not all, private insurance carriers may also cover monitor costs. CR advises patients to check which brands of CGM and supplies their insurance covers. Detail is important here. For example, the Dexcom G6 CGM System requires Dexcom G6 sensors (each lasts 10 days; 36 needed for almost 1 year), Dexcom G6 transmitters (each lasts up to three months; an estimated four needed/year), and a compatible smart phone or touchscreen device.5 According to Amazon,6 Dexcom G6 annual supplies cost $4,744/year. If a patient enrolls in an annual subscription for CGM supplies and is billed automatically to the patient’s credit card, they can get four free transmitters over the annual subscription period. This results in a $299 fee each month, and a discounted annual cost of $3,588 (Table 3).
[Note: A representative reached out on behalf of Dexcom to clarify some points with regard to insurance coverage of their CGM technologies after the publication of this article. This note is shared to help clarify any confusion this article may have caused.
The representative stated, “Dexcom is covered by 99 percent of private insurance providers in the U.S., in addition to Medicare, and has strong Medicaid coverage for low-income families in more than 40 states. It’s important that, no matter a patient’s financial situation, they have access to this life-changing technology, and Dexcom is committed to that mission. In 2020, Dexcom also launched a new process with the VA to help increase access to CGM among the veteran diabetes population through VA pharmacies. Additionally, 70 percent of pharmacy patients pay less than $60 per month, and 30 percent of those patients pay $0 out-of-pocket.”]
The cost of a CGM is an obstacle to patient adoption due to high-deductible insurance plans and copays for patients who must monitor blood glucose multiple times per day and night (that is, if coverage is even available at all).2 With more competition emerging, CGM costs are decreasing, which will improve market access.
The Medi-Vantage Perspective
Dr. Bogdanovics heralded the trend of increased use of CGMs and offered market share estimations. “In my practice, 85 percent of anybody on insulin has a CGM, but if you look across the nation, the device penetration is well below 50 percent—probably somewhere around 35 percent,” he said.1 “There’s still a lot of room to improve the use of it.”
Dr. Bogdanovics believes glucose-strip based BGM will decline. “I think CGM is here to stay, and it’s improving,” he said. “[Smart devices are] taking off, but I think that we’ve just got to get the message out there,” Dr. Bogdanovics concluded.
References
Maria Shepherd has more than 20 years of leadership experience in medical device/life-science marketing in small startups and top-tier companies. After her industry career, she founded Medi-Vantage. Medi-Vantage provides marketing and business strategy and innovation research for the medical device industry. The firm quantitatively and qualitatively sizes and segments opportunities, evaluates new technologies, provides marketing services, and assesses prospective acquisitions. Shepherd can be reached at mshepherd@medi-vantage.com. Visit her website at www.medi-vantage.com.
The change in technology is impressive. “Decades ago, we were still checking urine sugars,” Dr. Egils Bogdanovics, a Connecticut-based endocrinologist, said in a recent article.1 “In the early 1980s, fingerstick blood glucose monitors came out. That was a big deal—a revolution in diabetes.”
FDA cleared the first CGM in 1999, and CGM technology has rapidly evolved. The most up-to-date CGMs sent data via Bluetooth to smart phones through the cloud, allowing patients to read their blood glucose levels through an app in real-time.
That standard of care is rapidly changing. According to a recent report,2 the CGM market is experiencing significant change with new trends that are expected to shift management of Type 1 and Type 2 for people with diabetes. CGM technology is improving with several new closed-loop systems expected to launch in the next year. The prediction has been made that traditional blood glucose meters will become obsolete in the face of closed loop CGM, which will see rapid growth as patients switch out old meters for new technology.
Telehealth may also be a CGM market driver if it is used to treat patients with diabetes by the care team. Virtual visits may decline to <50 percent of office visits post-COVID but are expected to remain high if integrated data access becomes the norm in telehealth platforms.
New CGM tech will change the way patients with diabetes manage their care. Fingerstick glucose monitoring provided readings that were only a snapshot in time. New CGMs will change all that. “Initially, the main emphasis was to avoid hypoglycemia,” said Bogdanovics.1 This was a big risk for patients with Type 1 diabetes, that they might not be aware that their blood sugar levels had dropped to hazardous levels, leaving them little time to manage the problem.
The newest CGMs will simplify the treatment of patients with diabetes because they are automated, closed loop, and require no finger sticks or need for calibration. However, all contain a warning—CGM IFUs state if glucose alerts and readings do not match symptoms or meet their expectations, patients should use a traditional blood glucose meter to make diabetes management decisions. Otherwise, new CGMs allow patients to read their glucose numbers with selected types of smart phones or devices. Patients can get alerts based on customized settings and share their glucose data with designated family, friends, or clinicians.
Why This Is Important
Market shares are changing based on the newest automated technologies, but in 2019, U.S. sales of private label blood glucose meters (BGM) were the largest segment, with manufacturer branded devices a distant second—$167.7 million vs. $122.4 million, respectively (Table 1).3 According to Statista, in 2019, the second best-selling blood glucose meter brand, after aggregated private label brands, was the Lifescan One Touch Ultra (formerly a Johnson & Johnson product but divested to Platinum Equity in 2018 for an estimated $2.1 billion4).
Walmart, CVS, and other market share holders will not give those sales up easily. These companies bring distribution power to the supplier of products for patients with diabetes, and emissaries are probably at the tables of all the manufacturers of CGM, like Medtronic, DexCom, and Insulet.
Diabetic Personal Care: A Big, Growing Market in the U.S.
Diabetes has grown to global epidemic proportions and the market for diabetic personal care will continue to accelerate. It is reported that 1.26 million blood glucose meters were sold in the U.S. in 2018,2 an increase from reports of 960,000 blood glucose meters sold in 2017 (Table 2). That is a shocking increase of 31 percent.
According to Consumer Reports (CR), patients should choose a new CGM based on the features, benefits, and options that fit with their needs. CR also recommends patients compare the retail price of a new CGM to the cost of the continued use of blood glucose monitoring test strips with old BGM tech. Test strips were the true profit driver in this market and cost $18 to $184 per package of 100. CR estimates, depending upon patient diagnosis, the test strips can be an additional $265 to $2,685 or more per year for patients with diabetes. Replacement lances are an additional cost, and under some plans, test strips coverage depends upon the patient’s insulin requirements.
Another factor a patient should consider is insurance. Medicare covers some diabetes-related supplies. Some, not all, private insurance carriers may also cover monitor costs. CR advises patients to check which brands of CGM and supplies their insurance covers. Detail is important here. For example, the Dexcom G6 CGM System requires Dexcom G6 sensors (each lasts 10 days; 36 needed for almost 1 year), Dexcom G6 transmitters (each lasts up to three months; an estimated four needed/year), and a compatible smart phone or touchscreen device.5 According to Amazon,6 Dexcom G6 annual supplies cost $4,744/year. If a patient enrolls in an annual subscription for CGM supplies and is billed automatically to the patient’s credit card, they can get four free transmitters over the annual subscription period. This results in a $299 fee each month, and a discounted annual cost of $3,588 (Table 3).
[Note: A representative reached out on behalf of Dexcom to clarify some points with regard to insurance coverage of their CGM technologies after the publication of this article. This note is shared to help clarify any confusion this article may have caused.
The representative stated, “Dexcom is covered by 99 percent of private insurance providers in the U.S., in addition to Medicare, and has strong Medicaid coverage for low-income families in more than 40 states. It’s important that, no matter a patient’s financial situation, they have access to this life-changing technology, and Dexcom is committed to that mission. In 2020, Dexcom also launched a new process with the VA to help increase access to CGM among the veteran diabetes population through VA pharmacies. Additionally, 70 percent of pharmacy patients pay less than $60 per month, and 30 percent of those patients pay $0 out-of-pocket.”]
The cost of a CGM is an obstacle to patient adoption due to high-deductible insurance plans and copays for patients who must monitor blood glucose multiple times per day and night (that is, if coverage is even available at all).2 With more competition emerging, CGM costs are decreasing, which will improve market access.
The Medi-Vantage Perspective
Dr. Bogdanovics heralded the trend of increased use of CGMs and offered market share estimations. “In my practice, 85 percent of anybody on insulin has a CGM, but if you look across the nation, the device penetration is well below 50 percent—probably somewhere around 35 percent,” he said.1 “There’s still a lot of room to improve the use of it.”
Dr. Bogdanovics believes glucose-strip based BGM will decline. “I think CGM is here to stay, and it’s improving,” he said. “[Smart devices are] taking off, but I think that we’ve just got to get the message out there,” Dr. Bogdanovics concluded.
References
- bit.ly/mpo210401
- SVBLeerink Diabetes Deep Dive - Conversation with KOLs
- bit.ly/mpo210403
- bit.ly/mpo210404
- bit.ly/mpo210405
- bit.ly/mpo210406
- bit.ly/mpo210407
Maria Shepherd has more than 20 years of leadership experience in medical device/life-science marketing in small startups and top-tier companies. After her industry career, she founded Medi-Vantage. Medi-Vantage provides marketing and business strategy and innovation research for the medical device industry. The firm quantitatively and qualitatively sizes and segments opportunities, evaluates new technologies, provides marketing services, and assesses prospective acquisitions. Shepherd can be reached at mshepherd@medi-vantage.com. Visit her website at www.medi-vantage.com.