Business Wire08.11.20
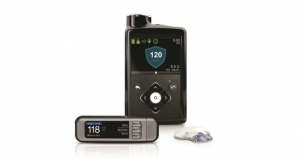
Tandem Diabetes Care Inc., an insulin delivery and diabetes technology company, announced U.S. Food and Drug Administration (FDA) clearance of an expanded pediatric indication for the t:slim X2 insulin pump with Control-IQ technology to children age six and older. The product was previously approved for ages 14 and older. Control-IQ technology is an advanced hybrid closed-loop feature designed to increase time in range (70-180 mg/dL).1
Control-IQ technology helps simplify diabetes management for younger patients by adjusting insulin delivery to help prevent highs and lows, automatically delivering correction boluses up to once per hour, and offering exercise and sleep-specific features. Integrated with Dexcom G6 continuous glucose monitoring (CGM), Control-IQ technology requires no fingersticks for calibration or diabetes treatment decisions.2
In a recent six-month study of children age 6 to 13 using the t:slim X2 pump with Control-IQ technology, sensor time in range (TIR) increased to 67 percent from 53 percent compared to those in the control group using sensor-augmented pump (SAP) alone (p<0.001). Overnight, children using Control-IQ technology in the same study stayed in range an average of 80 percent of the time, compared to 54 percent in the control group. This data from the International Diabetes Closed Loop Protocol-5 (DCLP5) study, funded by the National Institutes of Health (NIH), was presented earlier this year at the 13th International Conference on Advanced Technologies and Treatments for Diabetes (ATTD) in Madrid, Spain.3
“Nearly 40,000 t:slim X2 users have updated their pump with our revolutionary Control-IQ technology,” said John Sheridan, president and CEO of Tandem Diabetes Care. “The overwhelmingly positive benefits that people report experiencing can now be offered to a broader group of children with diabetes, which is particularly important as younger people often struggle with their glucose control.”
Benefits of Control-IQ Advanced Hybrid Closed-Loop Technology:
Standard Features of the t:slim X2 Insulin Pump:
Diabetes is a chronic, life-threatening disease that affects more than 30 million people in the United States, or nearly 1 in 10 Americans. Tandem estimates that more than three million people in the United States require daily administration of insulin and are candidates for pump therapy. More than 500,000 Americans with type 1 diabetes use an insulin pump, or approximately 30 percent of the type 1 diabetes population. In addition, approximately 100,000 Americans with type 2 diabetes use an insulin pump, which is less than 10 percent of the type 2 diabetes population using intensive insulin therapy management.
References
1 As measured by CGM
2 Zero fingersticks required when using the t:slim X2 pump with Dexcom G6 CGM integration. If glucose alerts and CGM readings do not match symptoms or expectations, use a blood glucose meter to make diabetes treatment decisions. Dexcom G6 CGM sold separately. Dexcom transmitter can only be paired with one medical device (either a Dexcom receiver or t:slim X2 pump) and one consumer device (phone or tablet) at the same time.
3 Wadwa RP. Impact of Control-IQ on glycemic control for school age children with T1D. Industry Symposium. ATTD, Madrid, Spain; February 20, 2020.
4 If glucose values are predicted to be above 180 mg/dL, Control-IQ technology calculates a correction bolus using the Personal Profile settings and a target of 110 mg/dL and delivers 60 percent of that value. It will do this up to once per hour as needed. An Automatic Correction Bolus will not occur within 60 minutes of a bolus that has been delivered or cancelled.
5 G6 readings can be used to make diabetes treatment decisions when taking up to a maximum acetaminophen dose of 1,000 mg every six hours. Taking a higher dose may affect the G6 readings.
6 Requires separate Dexcom Follow app.
7 Thirty-eight percent smaller than MiniMed 630G and 670G and at least 28 percent smaller than MiniMed 530G, Animas Vibe, and Omnipod System. Data on file, Tandem Diabetes Care.
Control-IQ technology helps simplify diabetes management for younger patients by adjusting insulin delivery to help prevent highs and lows, automatically delivering correction boluses up to once per hour, and offering exercise and sleep-specific features. Integrated with Dexcom G6 continuous glucose monitoring (CGM), Control-IQ technology requires no fingersticks for calibration or diabetes treatment decisions.2
In a recent six-month study of children age 6 to 13 using the t:slim X2 pump with Control-IQ technology, sensor time in range (TIR) increased to 67 percent from 53 percent compared to those in the control group using sensor-augmented pump (SAP) alone (p<0.001). Overnight, children using Control-IQ technology in the same study stayed in range an average of 80 percent of the time, compared to 54 percent in the control group. This data from the International Diabetes Closed Loop Protocol-5 (DCLP5) study, funded by the National Institutes of Health (NIH), was presented earlier this year at the 13th International Conference on Advanced Technologies and Treatments for Diabetes (ATTD) in Madrid, Spain.3
“Nearly 40,000 t:slim X2 users have updated their pump with our revolutionary Control-IQ technology,” said John Sheridan, president and CEO of Tandem Diabetes Care. “The overwhelmingly positive benefits that people report experiencing can now be offered to a broader group of children with diabetes, which is particularly important as younger people often struggle with their glucose control.”
Benefits of Control-IQ Advanced Hybrid Closed-Loop Technology:
- Predicts and helps prevent lows and highs – Control-IQ technology uses CGM readings to predict glucose values 30 minutes ahead. If glucose values are predicted to drop below 112.5 mg/dL, basal insulin delivery is reduced, and when predicted to be below 70 mg/dL, basal insulin delivery is stopped. If glucose values are predicted to be above 160 mg/dL in the next 30 minutes, basal insulin will be increased to help keep glucose in range (70-180 mg/dL).1
- Automatic Correction Boluses – If glucose values are predicted to be above 180 mg/dL, Control-IQ technology calculates a correction bolus with a target of 110 mg/dL and delivers 60 percent of that value. It will do this up to once an hour as needed.4
- Accommodates for sleep and exercise – Control-IQ technology offers optional settings for sleep and exercise that change the treatment values to better match the different physiologic needs during these activities.
- No fingersticks – With Dexcom G6 CGM integration, the Control-IQ feature works with no fingersticks required for mealtime dosing or calibration.2 Other benefits of the Dexcom G6 CGM include an extended 10-day wear, acetaminophen blocking,5 and the ability to share real-time CGM data with up to 10 followers.6
Standard Features of the t:slim X2 Insulin Pump:
- Color touchscreen – The large color touchscreen on the t:slim X2 pump is easy to read, simple to learn, and intuitive to use for anyone familiar with a smartphone or tablet.
- Small and discreet – The t:slim X2 pump is up to 38 percent smaller than other pumps,7 yet can hold up to 300-units of insulin.
- Can be used with or without the Control-IQ feature or CGM – When advanced features are turned off, the t:slim X2 pump removes the CGM chart from the screen and puts the Bolus and Option buttons front and center for easy access.
Diabetes is a chronic, life-threatening disease that affects more than 30 million people in the United States, or nearly 1 in 10 Americans. Tandem estimates that more than three million people in the United States require daily administration of insulin and are candidates for pump therapy. More than 500,000 Americans with type 1 diabetes use an insulin pump, or approximately 30 percent of the type 1 diabetes population. In addition, approximately 100,000 Americans with type 2 diabetes use an insulin pump, which is less than 10 percent of the type 2 diabetes population using intensive insulin therapy management.
References
1 As measured by CGM
2 Zero fingersticks required when using the t:slim X2 pump with Dexcom G6 CGM integration. If glucose alerts and CGM readings do not match symptoms or expectations, use a blood glucose meter to make diabetes treatment decisions. Dexcom G6 CGM sold separately. Dexcom transmitter can only be paired with one medical device (either a Dexcom receiver or t:slim X2 pump) and one consumer device (phone or tablet) at the same time.
3 Wadwa RP. Impact of Control-IQ on glycemic control for school age children with T1D. Industry Symposium. ATTD, Madrid, Spain; February 20, 2020.
4 If glucose values are predicted to be above 180 mg/dL, Control-IQ technology calculates a correction bolus using the Personal Profile settings and a target of 110 mg/dL and delivers 60 percent of that value. It will do this up to once per hour as needed. An Automatic Correction Bolus will not occur within 60 minutes of a bolus that has been delivered or cancelled.
5 G6 readings can be used to make diabetes treatment decisions when taking up to a maximum acetaminophen dose of 1,000 mg every six hours. Taking a higher dose may affect the G6 readings.
6 Requires separate Dexcom Follow app.
7 Thirty-eight percent smaller than MiniMed 630G and 670G and at least 28 percent smaller than MiniMed 530G, Animas Vibe, and Omnipod System. Data on file, Tandem Diabetes Care.