U.S. Food and Drug Administration10.06.21
Medtronic updated the below recall (which was made public in February 2020) with information that the company will replace any MiniMed 600 series insulin pump with a clear retainer ring with one containing the updated black retainer ring at no charge. A replacement insulin pump will be provided even if the clear retainer ring is not damaged and regardless of the pump’s warranty status.
The original recall announcement is below.
The FDA has identified this as a Class I recall, the most serious type of recall. Use of these devices may cause serious injuries or death.
Recalled Product
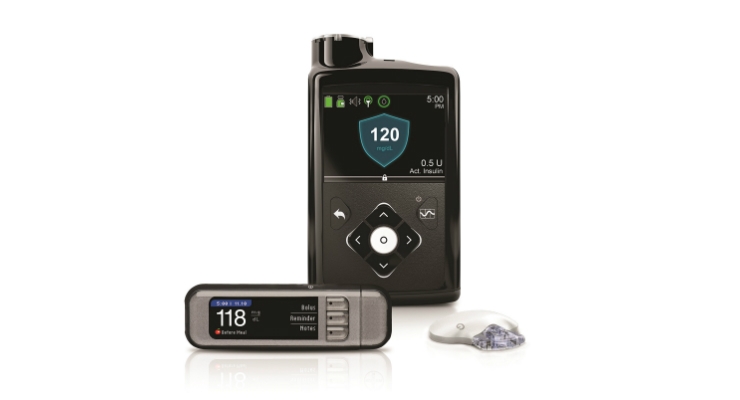
MiniMed 600 Series Insulin Pumps:
People who have Type 1 diabetes may use the MiniMed insulin pump to deliver insulin for the management of their diabetes. The Model 630G insulin pump may be used by persons sixteen years of age and older. The Model 670G insulin pump may be used by persons fourteen years of age and older.
Medtronic is recalling the specified insulin pumps due to a missing or broken retainer ring which helps to lock the insulin cartridge into place in the pump's reservoir compartment. If the cartridge is not locked firmly into place, under or over delivery of insulin may occur, which could result in hypoglycemia or hyperglycemia. Severe hyperglycemia can result in a loss of consciousness, seizure, and death.
The firm has received a total of 26,421 complaints in which the device malfunctioned in this manner. The firm is aware of 2,175 injuries and 1 death.
Who May Be Affected
Any person with diabetes who uses an affected Medtronic MiniMed insulin pump or health care providers who treat people with diabetes using the affected MiniMed insulin pumps.
On November 21, 2019 Medtronic notified affected customers and advised them to:
Customers who have questions or need additional information or support about this recall should call the 24-hour Medtronic Technical Support at 877-585-0166.
The original recall announcement is below.
The FDA has identified this as a Class I recall, the most serious type of recall. Use of these devices may cause serious injuries or death.
Recalled Product
MiniMed 600 Series Insulin Pumps:
- Model 630G (MMT-1715) - all lots before October 2019
- Model 670G (MMT-1780) - all lots before August 2019
- Model 630G Distribution Dates: September 2016 to October 2019
- Model 670G Distribution Dates: June 2017 to August 2019
- Date Initiated by Firm: November 21, 2019
People who have Type 1 diabetes may use the MiniMed insulin pump to deliver insulin for the management of their diabetes. The Model 630G insulin pump may be used by persons sixteen years of age and older. The Model 670G insulin pump may be used by persons fourteen years of age and older.
Medtronic is recalling the specified insulin pumps due to a missing or broken retainer ring which helps to lock the insulin cartridge into place in the pump's reservoir compartment. If the cartridge is not locked firmly into place, under or over delivery of insulin may occur, which could result in hypoglycemia or hyperglycemia. Severe hyperglycemia can result in a loss of consciousness, seizure, and death.
The firm has received a total of 26,421 complaints in which the device malfunctioned in this manner. The firm is aware of 2,175 injuries and 1 death.
Who May Be Affected
Any person with diabetes who uses an affected Medtronic MiniMed insulin pump or health care providers who treat people with diabetes using the affected MiniMed insulin pumps.
On November 21, 2019 Medtronic notified affected customers and advised them to:
- Examine the retainer ring of their pump.
- Stop use of the pump and contact Medtronic for a replacement pump if the reservoir does not lock into the pump or if the retainer ring is loose, damaged, or missing. If you stop using the pump, you should follow your doctor's recommendations and perform manual insulin injections.
- Continue using the pump if the reservoir locks in place correctly.
- If the pump is dropped by accident, check the pump and retainer ring for damage and stop use if it is damaged.
- Check the pump retainer ring and verify that the reservoir is locked correctly at every set change.
Customers who have questions or need additional information or support about this recall should call the 24-hour Medtronic Technical Support at 877-585-0166.