Maria Shepherd, President and Founder, Medi-Vantage05.01.19
For a long time, we all assumed Minnesota dominated the medical device industry worldwide, but is this still true? And, which other clusters lead in their contributions to the medtech industry?
Why This Is Important
When an industry cluster’s geography shifts, the ripple effect can be significant. Jobs are on the line as well as state revenues from taxes. The Compare Minnesota database tool shows California has surged past Minnesota in medical device employment and establishments.1 In addition, Massachusetts, long considered a premiere medtech hub, has been surpassed by Florida, Indiana, Pennsylvania, and New York (Table 1). How did this happen?
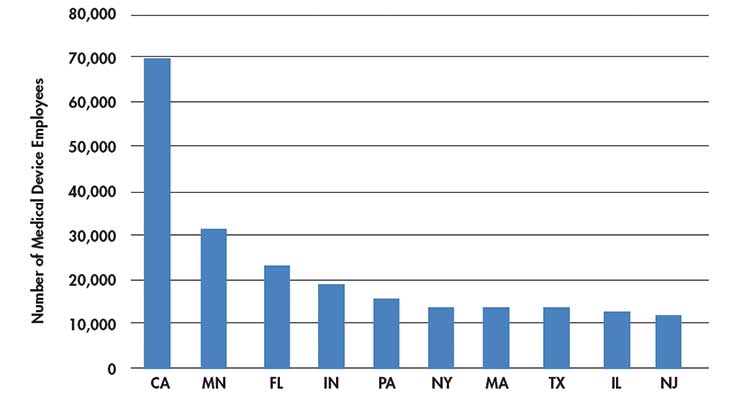
Table 1: Number of medtech employees.1
Medical Device Innovation in California Has Exploded
The Compare Minnesota database also shows how many medical device patents have emerged from each U.S. state.2 Patents are a sign of where the brainpower for the medical device industry actually lives, and once again, California beats Minnesota by approximately three times with 3,189 medtech patents. This is followed by Minnesota’s 991 patents and Massachusetts’s 810 patents (Table 2).
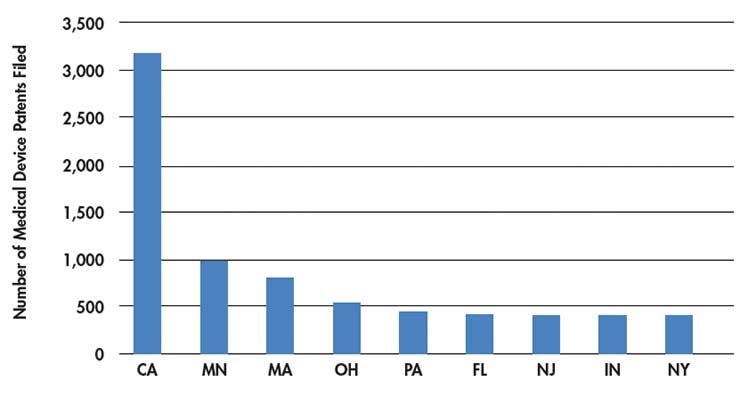
Table 2: Medical device patents by state.2
California is split into four major regions:
The next cluster of 400 to 500 patents per state begins with Ohio, Pennsylvania, Florida, New Jersey, Indiana, and New York.
Minnesota’s 991 patents in 2017 came from small and large medical device companies. There are many well-known medical device companies with a huge footprint in Minnesota—Boston Scientific, Abbott, and Medtronic, to name a few. Supporting industries for medical devices also thrive. Minnesota has a large network of medical device designers, contract manufacturers, medtech testing companies, and regulatory/quality consultants to support its medtech industry.
Massachusetts may have dropped in medical device industry employment, but it still ranks in the top three medical device states because many large medtech companies make their headquarters in Massachusetts. Massachusetts captures a large number of public and private dollars annually spent on innovation. Top research hospitals including Massachusetts General Hospital, Harvard Medical School, and many others received National Institutes of Health grants totaling $2.7 billion, according to a report sponsored by MassMEDIC.4
Only California leads Massachusetts when it comes to the number of FDA 510(k) clearances. Massachusetts ranked second among all states for 510(k)-cleared medical device patents and PMA approvals.
Florida ranks at the top for its quantity of medical device manufacturing facilities, according to the Enterprise Florida database.5 There are medtech companies all over the state, which also boasts an impressive number of incubators and accelerators.
Indiana is home to the orthopedics industry and to Cook Medical, a large, privately held medical device company. Indiana also hosts multiple medtech manufacturing centers. Pennsylvania benefits from its location near medical device companies that have their headquarters in New York and New Jersey.
Venture Capital Is Following the Brainpower
While California still leads in the number of VC investment dollars, New York is the runner up, followed by Massachusetts2 (Table 3). New York wants to get in on the action of being a medtech hub and state officials have lofty goals to give Massachusetts a run for its money as a life sciences innovation hub. In 2016, New York Gov. Andrew Cuomo announced a significant investment of $650 million to drive the growth of a world-class life sciences cluster in the Empire State. These funds are intended to grow the state’s capacity to commercialize research and expand its economy. New York is putting its money where its mouth is. This program includes tax incentives of $250 million for new and existing life sciences companies, state capital grants of $200 million to develop wet-lab and innovation space, and investment capital of $100 million for early-stage life science initiatives, with a match of at least $100 million for operating support from the private sector.
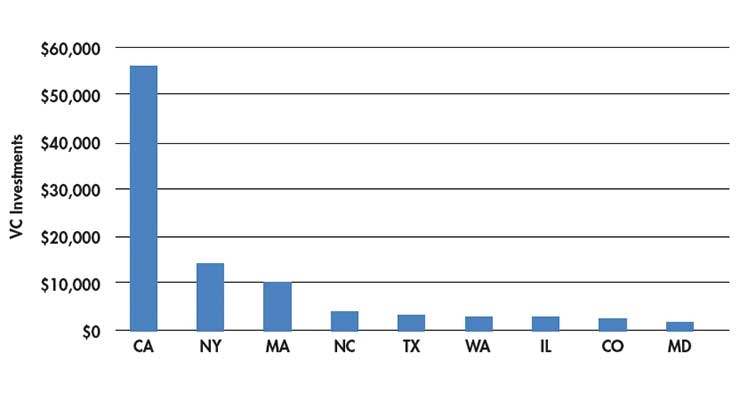
Table 3: Total venture capital investments by state (in millions).2
The Medi-Vantage Perspective
The takeaway from Table 3 is that VCs follow the money. Otherwise, how could New York have jumped to second place in VC investments? This is notable for other states that wish to maintain their standing in the medtech world—invest, or watch the investment money go elsewhere.
References
Maria Shepherd has more than 20 years of leadership experience in medical device/life science marketing in small startups and top-tier companies. After her industry career, including her role as vice president of marketing for Oridion Medical, where she boosted the company valuation prior to its acquisition by Covidien/Medtronic, director of marketing for Philips Medical, and senior management roles at Boston Scientific Corp., she founded Medi-Vantage. Medi-Vantage provides marketing, business strategy, and innovation research for the medical device, diagnostic, and digital health industries. The firm quantitatively and qualitatively sizes and segments opportunities, evaluates new technologies, provides marketing services, and assesses prospective acquisitions. Shepherd has taught marketing and product development courses and is a member of the Aligo Medtech Investment Committee (www.aligo.com). She can be reached at 855-343-3100. Visit her website at www.medi-vantage.com.
Why This Is Important
When an industry cluster’s geography shifts, the ripple effect can be significant. Jobs are on the line as well as state revenues from taxes. The Compare Minnesota database tool shows California has surged past Minnesota in medical device employment and establishments.1 In addition, Massachusetts, long considered a premiere medtech hub, has been surpassed by Florida, Indiana, Pennsylvania, and New York (Table 1). How did this happen?

Table 1: Number of medtech employees.1
Medical Device Innovation in California Has Exploded
The Compare Minnesota database also shows how many medical device patents have emerged from each U.S. state.2 Patents are a sign of where the brainpower for the medical device industry actually lives, and once again, California beats Minnesota by approximately three times with 3,189 medtech patents. This is followed by Minnesota’s 991 patents and Massachusetts’s 810 patents (Table 2).

Table 2: Medical device patents by state.2
California is split into four major regions:
- North of San Francisco
- Silicon Valley
- The Los Angeles metro area
- San Diego
The next cluster of 400 to 500 patents per state begins with Ohio, Pennsylvania, Florida, New Jersey, Indiana, and New York.
Minnesota’s 991 patents in 2017 came from small and large medical device companies. There are many well-known medical device companies with a huge footprint in Minnesota—Boston Scientific, Abbott, and Medtronic, to name a few. Supporting industries for medical devices also thrive. Minnesota has a large network of medical device designers, contract manufacturers, medtech testing companies, and regulatory/quality consultants to support its medtech industry.
Massachusetts may have dropped in medical device industry employment, but it still ranks in the top three medical device states because many large medtech companies make their headquarters in Massachusetts. Massachusetts captures a large number of public and private dollars annually spent on innovation. Top research hospitals including Massachusetts General Hospital, Harvard Medical School, and many others received National Institutes of Health grants totaling $2.7 billion, according to a report sponsored by MassMEDIC.4
Only California leads Massachusetts when it comes to the number of FDA 510(k) clearances. Massachusetts ranked second among all states for 510(k)-cleared medical device patents and PMA approvals.
Florida ranks at the top for its quantity of medical device manufacturing facilities, according to the Enterprise Florida database.5 There are medtech companies all over the state, which also boasts an impressive number of incubators and accelerators.
Indiana is home to the orthopedics industry and to Cook Medical, a large, privately held medical device company. Indiana also hosts multiple medtech manufacturing centers. Pennsylvania benefits from its location near medical device companies that have their headquarters in New York and New Jersey.
Venture Capital Is Following the Brainpower
While California still leads in the number of VC investment dollars, New York is the runner up, followed by Massachusetts2 (Table 3). New York wants to get in on the action of being a medtech hub and state officials have lofty goals to give Massachusetts a run for its money as a life sciences innovation hub. In 2016, New York Gov. Andrew Cuomo announced a significant investment of $650 million to drive the growth of a world-class life sciences cluster in the Empire State. These funds are intended to grow the state’s capacity to commercialize research and expand its economy. New York is putting its money where its mouth is. This program includes tax incentives of $250 million for new and existing life sciences companies, state capital grants of $200 million to develop wet-lab and innovation space, and investment capital of $100 million for early-stage life science initiatives, with a match of at least $100 million for operating support from the private sector.

Table 3: Total venture capital investments by state (in millions).2
The Medi-Vantage Perspective
The takeaway from Table 3 is that VCs follow the money. Otherwise, how could New York have jumped to second place in VC investments? This is notable for other states that wish to maintain their standing in the medtech world—invest, or watch the investment money go elsewhere.
References
- http://bit.ly/mpo190501
- http://bit.ly/mpo190502
- http://bit.ly/mpo190503 [PDF]
- http://bit.ly/mpo190504
- http://bit.ly/mpo190505 [PDF]
Maria Shepherd has more than 20 years of leadership experience in medical device/life science marketing in small startups and top-tier companies. After her industry career, including her role as vice president of marketing for Oridion Medical, where she boosted the company valuation prior to its acquisition by Covidien/Medtronic, director of marketing for Philips Medical, and senior management roles at Boston Scientific Corp., she founded Medi-Vantage. Medi-Vantage provides marketing, business strategy, and innovation research for the medical device, diagnostic, and digital health industries. The firm quantitatively and qualitatively sizes and segments opportunities, evaluates new technologies, provides marketing services, and assesses prospective acquisitions. Shepherd has taught marketing and product development courses and is a member of the Aligo Medtech Investment Committee (www.aligo.com). She can be reached at 855-343-3100. Visit her website at www.medi-vantage.com.