Abbott Laboratories03.19.19
Abbott announced positive late-breaking data from two separate analyses of the COAPT Trial showing additional key benefits from treatment with MitraClip for heart failure patients with clinically significant secondary (or functional) mitral regurgitation (MR), or leaky heart valve. Both data sets were presented at the American College of Cardiology's (ACC) 68th Annual Scientific Session. On Thursday, the U.S. Food and Drug Administration (FDA) approved a new, expanded indication for MitraClip to treat secondary mitral regurgitation based on the COAPT Trial data.
During the late-breaking session at ACC, MitraClip was shown to be superior to guideline-directed medical therapy in providing quality-of-life improvements for select patients with significant secondary MR resulting from advanced heart failure. These data were simultaneously published in the Journal of the American College of Cardiology. A second sub-analysis measured baseline characteristics of heart failure patients prior to enrollment in the COAPT Trial and indicated that all subgroups of patients enrolled in the trial benefited from MitraClip therapy in the long-term.
"Following the groundbreaking data from the COAPT Trial presented last September, these analyses further confirm that treatment with MitraClip provides great benefit for select patients with advanced heart failure," said Gregg W. Stone, M.D., co-principal investigator of the COAPT Trial, director of cardiovascular research and education at NewYork-Presbyterian/Columbia University Irving Medical Center and professor of medicine at Columbia University Vagelos College of Physicians and Surgeons. "In patients who remained symptomatic with moderate-to-severe or severe MR, despite all the best medical treatments, MitraClip prolonged their survival and markedly reduced the need for hospitalizations while improving their daily lives—despite advanced age and comorbidities."
People with heart failure may develop secondary MR when the left chamber of the heart becomes enlarged, preventing the mitral leaflets from closing and allowing blood to flow backwards through the heart.1 Significant secondary MR is difficult to manage, is associated with a poor prognosis,2 and can lead to reduced quality of life, recurrent hospitalizations and decreased survival.3,4 Most heart failure patients with clinically significant secondary MR are treated with medication only and have few treatment options.5 However, based on the recent approval, these patients can now benefit from treatment with MitraClip for their secondary MR.
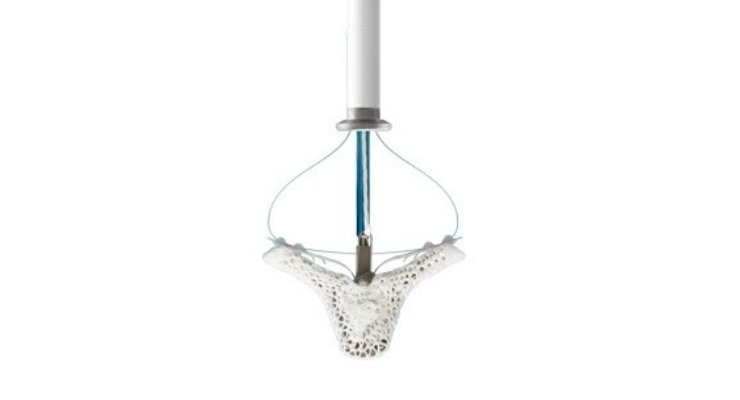
MitraClip is a small, clip-based device that repairs MR without the need for open-heart surgery. It is delivered to the heart through a small incision in the leg. The device works by clipping together a portion of the leaflets of the mitral valve to reduce the backflow of blood, which allows the heart to pump blood more efficiently. Approximately one in 10 adults age 75 and older in the U.S., or four million Americans, suffer from MR.6,7,8 It's estimated that two to three times as many patients may now benefit from MitraClip treatment for secondary MR as a result of underlying heart failure than those treated for the primary form of the disease commonly associated with the deterioration of the valve structure.9
"These additional analyses from the landmark COAPT Trial point to the advantages of MitraClip treatment for patients with severe MR who are not benefitting from medical therapy," said Michael Dale, vice president of Abbott's structural heart business. "The data underscore our recent approval to help these people who desperately need treatment, giving them the ability to do the things many of us take for granted: things like breathing normally, lying down to sleep and walking to the mailbox."
Quality-of-Life Data
The COAPT quality-of-life analysis showed that, at 24 months, patients with heart failure and secondary MR receiving maximally-tolerated medical therapy and treated with MitraClip demonstrated substantial and sustained health status improvement (≥10 points) compared with medical therapy (36.4 percent vs. 16.6 percent; p<0.001). While quality-of-life was unchanged over time in the medical therapy group, patients treated with MitraClip demonstrated substantial improvement in the KCCQ-OS score, a self-assessment of social abilities, symptoms, and quality-of-life, as soon as one month after the procedure (mean between-group difference 15.9 points, p<0.001), a difference which persisted through 24 months. The quality-of-life benefit of MitraClip was consistent across all subgroups through 24 months.
The COAPT trial randomized patients with ASE (American Society of Echocardiography) classification 3-4+ (moderate-to-severe to severe) secondary MR to treatment with MitraClip plus medical therapy (n=302) or medical therapy alone (n=312). At baseline, patients in both groups had substantially impaired quality-of-life due to their advanced heart failure. Quality-of-life was assessed at baseline and at one, six, 12 and 24 months.
Echocardiographic Sub-Analysis of COAPT Trial Data
In the COAPT imaging sub-analysis, patients were screened to assess the severity of secondary MR prior to enrollment in the trial, and to determine which patient characteristics best predict favorable long-term outcomes with MitraClip. A specific imaging protocol was developed and employed to select patients with severe MR who might benefit from the MitraClip device. A substantial reduction in death and heart failure hospitalizations was seen in all imaging subgroups regardless of patients' baseline characteristics.
In the sub-analysis, there were 614 patients with heart failure and ASE class 3+ or 4+ secondary MR enrolled and randomized 1:1 to MitraClip and medical therapy or medical therapy alone based on an integrated assessment of the severity of regurgitation using multiple measures and parameters. Follow-up cardiac imaging was obtained at discharge, one, six, 12, 18 and 24 months, and yearly through five years. Clinical follow-up is currently complete through one year in all patients and through two years in many of the patients.
About the COAPT Trial
In the COAPT (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation) Trial, 614 symptomatic heart failure patients with moderate-to-severe or severe secondary MR were randomized to receive treatment with MitraClip plus guideline-directed medical therapy or guideline-directed medical therapy alone at 78 sites in the U.S. and Canada. Eligible patients had diseased heart muscle, known as dilated cardiomyopathy that reduced the amount of blood pumped from the left ventricle; and moderate-to-severe or severe MR assessed by the American Society of Echocardiography guidelines that remained symptomatic despite maximally-tolerated medical therapy and cardiac resynchronization therapy (if appropriate).10,11 Mean patient age was 72.2 years, and 64 percent were male.
The primary efficacy endpoint in the COAPT Trial was all heart failure hospitalizations through two years, and the primary safety endpoint was freedom from device-related complications at one year compared to a performance goal of 88 percent. Secondary endpoints included all-cause mortality at two years, change in quality-of-life at one year, change in functional capacity (six minute walk distance) at one year, MR severity at one year and left ventricle size at one year. The COAPT Trial met its primary endpoints and all 10 secondary endpoints as presented during the TCT cardiology meeting in Sept. 2018 and published in the New England Journal of Medicine.12
References
1 Asgar AW, Mack MJ, Stone GW. Secondary mitral regurgitation in heart failure: pathophysiology, prognosis, and therapeutic considerations. J Am Coll Cardiol 2015;65:1231-48.
2 Dwivedi A, Vainrib A, Saric M. Functional mitral regurgitation in patients with heart failure and depressed ejection fraction. Current Opinion in Cardiology. September 2016 - Volume 31 - Issue 5 - p 483–492.
3 Sannino A, Smith II RL, Schiattarrella GG, et al. Survival and cardiovascular outcomes of patients with secondary mitral regurgitation: a meta-analysis of 53 studies. JAMA Cardiology 2017; 2:1130-39.
4 Goliasch G, Bartko PE, Pavo N, et al. Refining the prognosis impact of functional mitral regurgitation in chronic heart failure. Eur Heart J 2018;39:39-46.
5 Goel SS, Bajaj N, Aggarwal B, et al. Prevalence and outcomes of unoperated patients with severe symptomatic mitral regurgitation and heart failure: comprehensive analysis to determine the potential role of MitraClip for this unmet need. J Am Coll Cardiol. 2014;63:185-6.
6 Dziaddzko et al, "Outcome and Undertreatment of Mitral Regurgitation: a Community Cohort Study", Lancet 2018: 391:960-69.
7 AHA Heart Disease and Stroke Statistics Update, Circulation 2017.
8 Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA /guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Caridol. 2017;70:776-803.
9 Goel SS, Bajaj N, Aggarwal B, et al. Prevalence and outcomes of unoperated patients with severe symptomatic mitral regurgitation and heart failure: comprehensive analysis to determine the potential role of MitraClip for this unmet need. J Am Coll Cardiol. 2014;63:185-6.
10 Pecini et al EHJ 2011; Asgar et al, JACC 2015; Nieminen et al, EHJ 2006; Patel et al, Journal of Cardiac Failure 2004.
11 Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 201;70:252-289.
12 Stone GW, et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N Engl J Med. 2018 Dec 13;379(24):2307-2318.
During the late-breaking session at ACC, MitraClip was shown to be superior to guideline-directed medical therapy in providing quality-of-life improvements for select patients with significant secondary MR resulting from advanced heart failure. These data were simultaneously published in the Journal of the American College of Cardiology. A second sub-analysis measured baseline characteristics of heart failure patients prior to enrollment in the COAPT Trial and indicated that all subgroups of patients enrolled in the trial benefited from MitraClip therapy in the long-term.
"Following the groundbreaking data from the COAPT Trial presented last September, these analyses further confirm that treatment with MitraClip provides great benefit for select patients with advanced heart failure," said Gregg W. Stone, M.D., co-principal investigator of the COAPT Trial, director of cardiovascular research and education at NewYork-Presbyterian/Columbia University Irving Medical Center and professor of medicine at Columbia University Vagelos College of Physicians and Surgeons. "In patients who remained symptomatic with moderate-to-severe or severe MR, despite all the best medical treatments, MitraClip prolonged their survival and markedly reduced the need for hospitalizations while improving their daily lives—despite advanced age and comorbidities."
People with heart failure may develop secondary MR when the left chamber of the heart becomes enlarged, preventing the mitral leaflets from closing and allowing blood to flow backwards through the heart.1 Significant secondary MR is difficult to manage, is associated with a poor prognosis,2 and can lead to reduced quality of life, recurrent hospitalizations and decreased survival.3,4 Most heart failure patients with clinically significant secondary MR are treated with medication only and have few treatment options.5 However, based on the recent approval, these patients can now benefit from treatment with MitraClip for their secondary MR.
MitraClip is a small, clip-based device that repairs MR without the need for open-heart surgery. It is delivered to the heart through a small incision in the leg. The device works by clipping together a portion of the leaflets of the mitral valve to reduce the backflow of blood, which allows the heart to pump blood more efficiently. Approximately one in 10 adults age 75 and older in the U.S., or four million Americans, suffer from MR.6,7,8 It's estimated that two to three times as many patients may now benefit from MitraClip treatment for secondary MR as a result of underlying heart failure than those treated for the primary form of the disease commonly associated with the deterioration of the valve structure.9
"These additional analyses from the landmark COAPT Trial point to the advantages of MitraClip treatment for patients with severe MR who are not benefitting from medical therapy," said Michael Dale, vice president of Abbott's structural heart business. "The data underscore our recent approval to help these people who desperately need treatment, giving them the ability to do the things many of us take for granted: things like breathing normally, lying down to sleep and walking to the mailbox."
Quality-of-Life Data
The COAPT quality-of-life analysis showed that, at 24 months, patients with heart failure and secondary MR receiving maximally-tolerated medical therapy and treated with MitraClip demonstrated substantial and sustained health status improvement (≥10 points) compared with medical therapy (36.4 percent vs. 16.6 percent; p<0.001). While quality-of-life was unchanged over time in the medical therapy group, patients treated with MitraClip demonstrated substantial improvement in the KCCQ-OS score, a self-assessment of social abilities, symptoms, and quality-of-life, as soon as one month after the procedure (mean between-group difference 15.9 points, p<0.001), a difference which persisted through 24 months. The quality-of-life benefit of MitraClip was consistent across all subgroups through 24 months.
The COAPT trial randomized patients with ASE (American Society of Echocardiography) classification 3-4+ (moderate-to-severe to severe) secondary MR to treatment with MitraClip plus medical therapy (n=302) or medical therapy alone (n=312). At baseline, patients in both groups had substantially impaired quality-of-life due to their advanced heart failure. Quality-of-life was assessed at baseline and at one, six, 12 and 24 months.
Echocardiographic Sub-Analysis of COAPT Trial Data
In the COAPT imaging sub-analysis, patients were screened to assess the severity of secondary MR prior to enrollment in the trial, and to determine which patient characteristics best predict favorable long-term outcomes with MitraClip. A specific imaging protocol was developed and employed to select patients with severe MR who might benefit from the MitraClip device. A substantial reduction in death and heart failure hospitalizations was seen in all imaging subgroups regardless of patients' baseline characteristics.
In the sub-analysis, there were 614 patients with heart failure and ASE class 3+ or 4+ secondary MR enrolled and randomized 1:1 to MitraClip and medical therapy or medical therapy alone based on an integrated assessment of the severity of regurgitation using multiple measures and parameters. Follow-up cardiac imaging was obtained at discharge, one, six, 12, 18 and 24 months, and yearly through five years. Clinical follow-up is currently complete through one year in all patients and through two years in many of the patients.
About the COAPT Trial
In the COAPT (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation) Trial, 614 symptomatic heart failure patients with moderate-to-severe or severe secondary MR were randomized to receive treatment with MitraClip plus guideline-directed medical therapy or guideline-directed medical therapy alone at 78 sites in the U.S. and Canada. Eligible patients had diseased heart muscle, known as dilated cardiomyopathy that reduced the amount of blood pumped from the left ventricle; and moderate-to-severe or severe MR assessed by the American Society of Echocardiography guidelines that remained symptomatic despite maximally-tolerated medical therapy and cardiac resynchronization therapy (if appropriate).10,11 Mean patient age was 72.2 years, and 64 percent were male.
The primary efficacy endpoint in the COAPT Trial was all heart failure hospitalizations through two years, and the primary safety endpoint was freedom from device-related complications at one year compared to a performance goal of 88 percent. Secondary endpoints included all-cause mortality at two years, change in quality-of-life at one year, change in functional capacity (six minute walk distance) at one year, MR severity at one year and left ventricle size at one year. The COAPT Trial met its primary endpoints and all 10 secondary endpoints as presented during the TCT cardiology meeting in Sept. 2018 and published in the New England Journal of Medicine.12
References
1 Asgar AW, Mack MJ, Stone GW. Secondary mitral regurgitation in heart failure: pathophysiology, prognosis, and therapeutic considerations. J Am Coll Cardiol 2015;65:1231-48.
2 Dwivedi A, Vainrib A, Saric M. Functional mitral regurgitation in patients with heart failure and depressed ejection fraction. Current Opinion in Cardiology. September 2016 - Volume 31 - Issue 5 - p 483–492.
3 Sannino A, Smith II RL, Schiattarrella GG, et al. Survival and cardiovascular outcomes of patients with secondary mitral regurgitation: a meta-analysis of 53 studies. JAMA Cardiology 2017; 2:1130-39.
4 Goliasch G, Bartko PE, Pavo N, et al. Refining the prognosis impact of functional mitral regurgitation in chronic heart failure. Eur Heart J 2018;39:39-46.
5 Goel SS, Bajaj N, Aggarwal B, et al. Prevalence and outcomes of unoperated patients with severe symptomatic mitral regurgitation and heart failure: comprehensive analysis to determine the potential role of MitraClip for this unmet need. J Am Coll Cardiol. 2014;63:185-6.
6 Dziaddzko et al, "Outcome and Undertreatment of Mitral Regurgitation: a Community Cohort Study", Lancet 2018: 391:960-69.
7 AHA Heart Disease and Stroke Statistics Update, Circulation 2017.
8 Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA /guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Caridol. 2017;70:776-803.
9 Goel SS, Bajaj N, Aggarwal B, et al. Prevalence and outcomes of unoperated patients with severe symptomatic mitral regurgitation and heart failure: comprehensive analysis to determine the potential role of MitraClip for this unmet need. J Am Coll Cardiol. 2014;63:185-6.
10 Pecini et al EHJ 2011; Asgar et al, JACC 2015; Nieminen et al, EHJ 2006; Patel et al, Journal of Cardiac Failure 2004.
11 Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 201;70:252-289.
12 Stone GW, et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N Engl J Med. 2018 Dec 13;379(24):2307-2318.