Abbott Laboratories03.15.19
Abbott announced it received approval from the U.S. Food and Drug Administration (FDA) for a new, expanded indication to its leading MitraClip device used to repair a leaky mitral valve without open-heart surgery. Supported by the results of the landmark COAPT Trial, MitraClip is the first transcatheter mitral valve intervention therapy approved to treat select heart failure patients with clinically significant secondary, or functional, mitral regurgitation (MR).
The MitraClip transcatheter clip-based therapy, available in the U.S. since 2013 and now on a third generation of product innovations, has been used to treat more than 80,000 patients worldwide over the last 10 years. The new, expanded indication addresses the secondary form of MR and significantly increases the amount of people with MR able to be treated with MitraClip. Based on this approval, Abbott will begin discussions with the Center for Medicare and Medicaid Services (CMS) and physician specialty societies to request a revision to the national coverage determination (NCD) that would expand Medicare coverage to include secondary MR patients.
"Since severe secondary MR is extremely difficult to manage and associated with a poor prognosis, people have historically had few options," said Neil Moat, M.D., chief medical officer of Abbott's structural heart business. "The expanded indication of MitraClip opens new doors for these ailing patients and can improve their quality of life and chance of survival despite their complex condition."
Approximately four million Americans suffer from MR, specifically one in 10 adults age 75 and older.1,2,3,4 It's estimated two to three times as many patients may benefit from MitraClip treatment for secondary MR as a result of underlying heart failure than those treated for the primary (or degenerative) form of the disease associated with the structure of the valve.5 People with heart failure may develop secondary MR when the left chamber of the heart becomes enlarged, preventing the mitral leaflets from closing normally and allowing blood to flow backwards through the heart.6
Significant secondary MR can lead to reduced quality of life, recurrent hospitalizations and decreased survival.7,8 Medication alone is the current standard of care for most heart failure patients with secondary MR, but this approach only helps manage the symptoms and does not address the underlying cause. MitraClip is now an effective treatment option for these ailing patients.
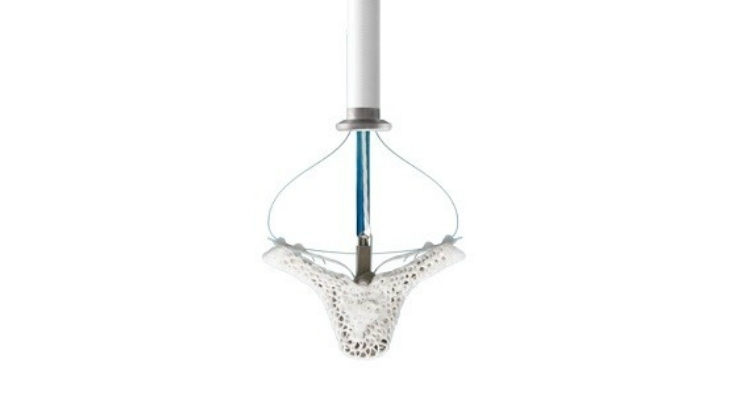
The MitraClip device repairs MR without open-heart surgery and is delivered to the heart through a small incision in the leg. The device clips portions of the leaflets, or flaps, of the mitral valve together to reduce the backflow of blood, restoring the heart's ability to pump oxygenated blood more efficiently. MitraClip provides almost immediate symptom relief and patients are released from the hospital on average after two days.
"Over the past decade, we have made significant and consistent investments to lead the development of novel treatments for mitral valve disease," said Michael Dale, vice president of Abbott's structural heart business. "The expanded indication for MitraClip is a direct result of the dedication of our employees as well as our remarkable clinical investigators."
Abbott is the global leader in developing transcatheter mitral valve technologies as alternatives to open-heart surgery. Building upon its success with the MitraClip device and many years of mitral valve experience and clinical evidence, Abbott is leading the way in novel, transcatheter devices by investing in the development of new mitral valve replacement options with its Tendyne and Cephea valve technologies.
References
1 Dziaddzko et al, "Outcome and Undertreatment of Mitral Regurgitation: a Community Cohort Study", Lancet 2018: 391:960-69.
2 AHA Heart Disease and Stroke Statistics Update, Circulation 2017.
3 Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Caridol. 2017;70:776-803.
4 Pecini et al EHJ 2011; Asgar et al, JACC 2015; Nieminen et al, EHJ 2006; Patel et al, Journal of Cardiac Failure 2004.
5 Goel SS, Bajaj N, Aggarwal B, et al. Prevalence and outcomes of unoperated patients with severe symptomatic mitral regurgitation and heart failure: comprehensive analysis to determine the potential role of MitraClip for this unmet need. J Am Coll Cardiol. 2014;63:185-6.
6 Asgar AW, Mack MJ, Stone GW. Secondary mitral regurgitation in heart failure: pathophysiology, prognosis, and therapeutic considerations. J Am Coll Cardiol 2015;65:1231-48.
7 Sannino A, Smith II RL, Schiattarrella GG, et al. Survival and cardiovascular outcomes of patients with secondary mitral regurgitation: a meta-analysis of 53 studies. JAMA Cardiology 2017; 2:1130-39.
8 Goliasch G, Bartko PE, Pavo N, et al. Refining the prognosis impact of functional mitral regurgitation in chronic heart failure. Eur Heart J 2018;39:39-46.
The MitraClip transcatheter clip-based therapy, available in the U.S. since 2013 and now on a third generation of product innovations, has been used to treat more than 80,000 patients worldwide over the last 10 years. The new, expanded indication addresses the secondary form of MR and significantly increases the amount of people with MR able to be treated with MitraClip. Based on this approval, Abbott will begin discussions with the Center for Medicare and Medicaid Services (CMS) and physician specialty societies to request a revision to the national coverage determination (NCD) that would expand Medicare coverage to include secondary MR patients.
"Since severe secondary MR is extremely difficult to manage and associated with a poor prognosis, people have historically had few options," said Neil Moat, M.D., chief medical officer of Abbott's structural heart business. "The expanded indication of MitraClip opens new doors for these ailing patients and can improve their quality of life and chance of survival despite their complex condition."
Approximately four million Americans suffer from MR, specifically one in 10 adults age 75 and older.1,2,3,4 It's estimated two to three times as many patients may benefit from MitraClip treatment for secondary MR as a result of underlying heart failure than those treated for the primary (or degenerative) form of the disease associated with the structure of the valve.5 People with heart failure may develop secondary MR when the left chamber of the heart becomes enlarged, preventing the mitral leaflets from closing normally and allowing blood to flow backwards through the heart.6
Significant secondary MR can lead to reduced quality of life, recurrent hospitalizations and decreased survival.7,8 Medication alone is the current standard of care for most heart failure patients with secondary MR, but this approach only helps manage the symptoms and does not address the underlying cause. MitraClip is now an effective treatment option for these ailing patients.
The MitraClip device repairs MR without open-heart surgery and is delivered to the heart through a small incision in the leg. The device clips portions of the leaflets, or flaps, of the mitral valve together to reduce the backflow of blood, restoring the heart's ability to pump oxygenated blood more efficiently. MitraClip provides almost immediate symptom relief and patients are released from the hospital on average after two days.
"Over the past decade, we have made significant and consistent investments to lead the development of novel treatments for mitral valve disease," said Michael Dale, vice president of Abbott's structural heart business. "The expanded indication for MitraClip is a direct result of the dedication of our employees as well as our remarkable clinical investigators."
Abbott is the global leader in developing transcatheter mitral valve technologies as alternatives to open-heart surgery. Building upon its success with the MitraClip device and many years of mitral valve experience and clinical evidence, Abbott is leading the way in novel, transcatheter devices by investing in the development of new mitral valve replacement options with its Tendyne and Cephea valve technologies.
References
1 Dziaddzko et al, "Outcome and Undertreatment of Mitral Regurgitation: a Community Cohort Study", Lancet 2018: 391:960-69.
2 AHA Heart Disease and Stroke Statistics Update, Circulation 2017.
3 Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Caridol. 2017;70:776-803.
4 Pecini et al EHJ 2011; Asgar et al, JACC 2015; Nieminen et al, EHJ 2006; Patel et al, Journal of Cardiac Failure 2004.
5 Goel SS, Bajaj N, Aggarwal B, et al. Prevalence and outcomes of unoperated patients with severe symptomatic mitral regurgitation and heart failure: comprehensive analysis to determine the potential role of MitraClip for this unmet need. J Am Coll Cardiol. 2014;63:185-6.
6 Asgar AW, Mack MJ, Stone GW. Secondary mitral regurgitation in heart failure: pathophysiology, prognosis, and therapeutic considerations. J Am Coll Cardiol 2015;65:1231-48.
7 Sannino A, Smith II RL, Schiattarrella GG, et al. Survival and cardiovascular outcomes of patients with secondary mitral regurgitation: a meta-analysis of 53 studies. JAMA Cardiology 2017; 2:1130-39.
8 Goliasch G, Bartko PE, Pavo N, et al. Refining the prognosis impact of functional mitral regurgitation in chronic heart failure. Eur Heart J 2018;39:39-46.