Vinisha Joshi, Research Content Developer, Global Market Insights04.01.21
Despite medical therapy making great progress over the years, cardiovascular diseases (CVDs) have remained a leading cause of fatalities worldwide. According to WHO estimates, CVDs cause over 17.9 million deaths annually, accounting for nearly 31 percent of deaths across the globe.
As the burden of the condition on the global medical landscape grows, cardiovascular devices are gaining rapid traction over the years, with the technologies making significant contributions to modern patient care.
For patients meeting the required criteria, therapy can be provided using different cardiovascular medical devices. These include ICDs (implantable cardioverter-defibrillators), cardiac ablation devices, LAA (left atrial appendage) closure devices, and EVH (endoscopic vessel harvesting) devices, among others.
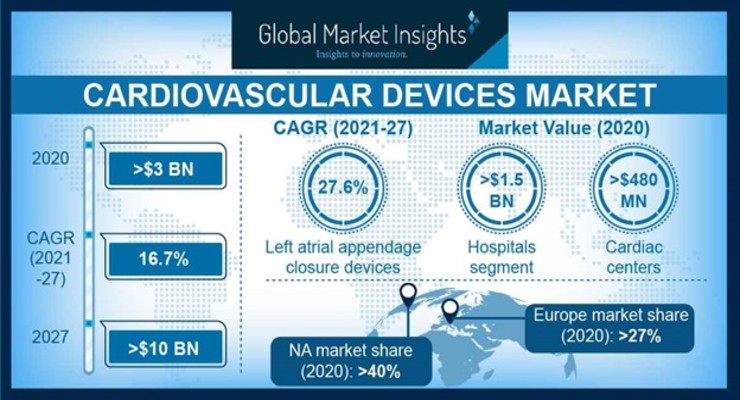
A report by Global Market Insights Inc. estimates the cardiovascular device market size is likely to exceed a valuation of $10 billion by 2027.
Miniaturization of Healthcare Devices
According to a Bloomberg Business prediction made way back in 2009, miniaturization of medical equipment was touted as one of the most significant innovations of the upcoming decade. In the modern era, this prediction is gradually being realized, as the emergence of convenient, portable consumer electronic devices triggers demand for the same functionality and convenience in medical devices.
Furthermore, increasing preference for shorter hospital stays and rising propensity for minimally invasive procedures have increased the need for advanced portable monitoring and diagnostic equipment that can remain with the patient even in recovery. Miniaturization is already a key feature in many other industries, which have laid the groundwork for the technology’s evolution in the medical domain, particularly in the cardiovascular device industry.
The advent of high-performance chips that can offer robust processing capacity at minuscule sizes—alongside microscopic sensors, large data storage capacity, wireless connectivity, and other technological advancements—are paving the way for a new generation of miniaturized cardiac devices.
These devices, many of which can be implanted directly into the heart, serve many purposes in CVD treatment. Pacing devices can eliminate abnormalities in heart rhythm and prevent low heart rates. Long-term rhythm monitoring devices can detect arrhythmias in unexplained syncope cases, and hemodynamic monitoring devices can identify incidents of heart failure.
The use of these miniaturized cardiovascular medical devices has been known to deliver prevention of hospitalization due to heart failure (HF), as well as an improvement in the life quality of patients.
In October 2020, preclinical-stage medical device company FineHeart SA, which developed an ICOMS (Implantable Cardiac Output Management System) designed to fulfill unmet needs of patients suffering from severe HF, revealed positive results from a 30-day preclinical study. It confirmed the device’s ability to deliver high hemodynamic performance with continuous and pulsed, increased cardiac output up to 6L/min, and considerably lower risk of thrombosis or hemolysis.
The ICOMS technology, an innovative cardiac assist device-pacemaker hybrid, was hailed as the first completely intraventricular flow accelerator to provide physiologic, pulsatile support to native heart function without an aortic bypass. It is implanted via a minimally invasive procedure that can be performed in under 90 minutes, and runs on a rechargeable wireless power source, mitigating rehospitalization or complication risks.
COVID-19 Triggers a Surge in Cardiovascular Incidences
While associated primarily with respiratory conditions, the novel coronavirus, which has triggered the deadly COVID-19 pandemic, has begun to show many more physiological manifestations as the number of cases grows. These range from complications in the brain, kidneys, and other organs, as well as a sizeable impact on the cardiovascular system.
In fact, estimates from an American College of Cardiology (ACC) report suggest over 40 percent of COVID-19 patients suffer from cardiovascular or cerebrovascular disease, with nearly 16.7 percent of patients developing arrhythmias. COVID-19 patients are also at high risk for fibrillation, which has shed light on the burgeoning importance of advanced cardiac devices, particularly in the current pandemic era.
For instance, in February 2021, Medtronic’s DiamondTemp Ablation (DTA) system received FDA approval to treat patients with recurrent symptomatic paroxysmal AF (atrial fibrillation) who have been unresponsive to drug therapies. The DTA catheter, an open-irrigated, temperature-controlled RF ablation system, has industrial-grade diamonds embedded in it. These allow 200-400 times higher thermal conductivity as compared to conventional RF ablation catheter materials.
GE Healthcare also obtained 510(k) clearance from the FDA last October for the Ultra Edition package on its Vivid cardiovascular ultrasound devices. These included new AI-powered features facilitate quicker, more consistent, and repeatable exams while reducing exam time via 99 percent accuracy, 80 percent fewer clicks, and lesser inter-operator variability.
As the burden of the condition on the global medical landscape grows, cardiovascular devices are gaining rapid traction over the years, with the technologies making significant contributions to modern patient care.
For patients meeting the required criteria, therapy can be provided using different cardiovascular medical devices. These include ICDs (implantable cardioverter-defibrillators), cardiac ablation devices, LAA (left atrial appendage) closure devices, and EVH (endoscopic vessel harvesting) devices, among others.
A report by Global Market Insights Inc. estimates the cardiovascular device market size is likely to exceed a valuation of $10 billion by 2027.
Miniaturization of Healthcare Devices
According to a Bloomberg Business prediction made way back in 2009, miniaturization of medical equipment was touted as one of the most significant innovations of the upcoming decade. In the modern era, this prediction is gradually being realized, as the emergence of convenient, portable consumer electronic devices triggers demand for the same functionality and convenience in medical devices.
Furthermore, increasing preference for shorter hospital stays and rising propensity for minimally invasive procedures have increased the need for advanced portable monitoring and diagnostic equipment that can remain with the patient even in recovery. Miniaturization is already a key feature in many other industries, which have laid the groundwork for the technology’s evolution in the medical domain, particularly in the cardiovascular device industry.
The advent of high-performance chips that can offer robust processing capacity at minuscule sizes—alongside microscopic sensors, large data storage capacity, wireless connectivity, and other technological advancements—are paving the way for a new generation of miniaturized cardiac devices.
These devices, many of which can be implanted directly into the heart, serve many purposes in CVD treatment. Pacing devices can eliminate abnormalities in heart rhythm and prevent low heart rates. Long-term rhythm monitoring devices can detect arrhythmias in unexplained syncope cases, and hemodynamic monitoring devices can identify incidents of heart failure.
The use of these miniaturized cardiovascular medical devices has been known to deliver prevention of hospitalization due to heart failure (HF), as well as an improvement in the life quality of patients.
In October 2020, preclinical-stage medical device company FineHeart SA, which developed an ICOMS (Implantable Cardiac Output Management System) designed to fulfill unmet needs of patients suffering from severe HF, revealed positive results from a 30-day preclinical study. It confirmed the device’s ability to deliver high hemodynamic performance with continuous and pulsed, increased cardiac output up to 6L/min, and considerably lower risk of thrombosis or hemolysis.
The ICOMS technology, an innovative cardiac assist device-pacemaker hybrid, was hailed as the first completely intraventricular flow accelerator to provide physiologic, pulsatile support to native heart function without an aortic bypass. It is implanted via a minimally invasive procedure that can be performed in under 90 minutes, and runs on a rechargeable wireless power source, mitigating rehospitalization or complication risks.
COVID-19 Triggers a Surge in Cardiovascular Incidences
While associated primarily with respiratory conditions, the novel coronavirus, which has triggered the deadly COVID-19 pandemic, has begun to show many more physiological manifestations as the number of cases grows. These range from complications in the brain, kidneys, and other organs, as well as a sizeable impact on the cardiovascular system.
In fact, estimates from an American College of Cardiology (ACC) report suggest over 40 percent of COVID-19 patients suffer from cardiovascular or cerebrovascular disease, with nearly 16.7 percent of patients developing arrhythmias. COVID-19 patients are also at high risk for fibrillation, which has shed light on the burgeoning importance of advanced cardiac devices, particularly in the current pandemic era.
For instance, in February 2021, Medtronic’s DiamondTemp Ablation (DTA) system received FDA approval to treat patients with recurrent symptomatic paroxysmal AF (atrial fibrillation) who have been unresponsive to drug therapies. The DTA catheter, an open-irrigated, temperature-controlled RF ablation system, has industrial-grade diamonds embedded in it. These allow 200-400 times higher thermal conductivity as compared to conventional RF ablation catheter materials.
GE Healthcare also obtained 510(k) clearance from the FDA last October for the Ultra Edition package on its Vivid cardiovascular ultrasound devices. These included new AI-powered features facilitate quicker, more consistent, and repeatable exams while reducing exam time via 99 percent accuracy, 80 percent fewer clicks, and lesser inter-operator variability.