Business Wire06.10.21
Neurescue, a medical device company developing cardiovascular solutions to improve the outcomes for emergency patients, announced the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance to market the company’s NEURESCUE device for temporary occlusion of large vessels, including patients requiring emergency control of hemorrhage. The company also announced the FDA approval of its Investigational Device Exemption (IDE) application to conduct a clinical trial of a novel cardiac arrest treatment indication.
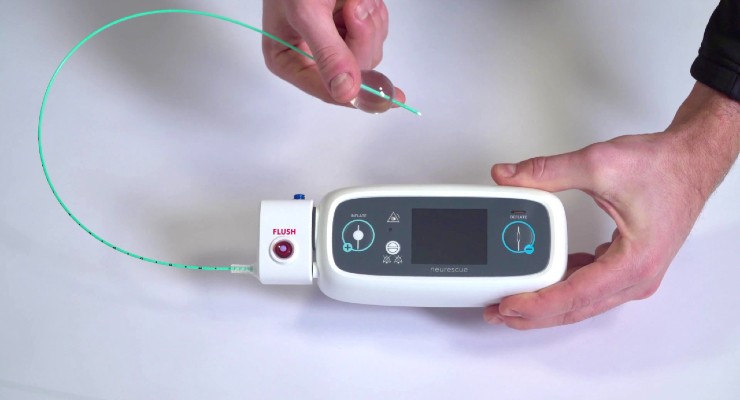
The NEURESCUE device is the world’s first intelligent balloon catheter for aortic occlusion, an emergency technique that supercharges blood flow to the heart and brain within one minute from deployment. The catheter-based device is delivered via the femoral artery, temporarily inflating a soft balloon in the aorta to redirect blood flow towards the upper body. The procedure is performed to provide additional time to control blood loss and bridge patients to additional life-saving treatment options.
"The NEURESCUE device represents a significant advancement that could meaningfully improve the survival rates for emergency patients,” said Maham Rahimi, M.D., Ph.D., assistant professor of cardiovascular surgery at Weill Cornell School of Medicine. “The device gives patients suffering traumatic blood loss a longer window of time to receive appropriate medical interventions, which can directly translate to saved lives.”
Hemorrhage is a substantial global unmet need, with more than 60,000 deaths per year in the U.S., and an estimated 1.9 million deaths per year worldwide, 1.5 million of which result from trauma, such as car accidents.1 Cardiac arrest represents an even larger unmet need, with more than half a million deaths each year in the U.S. alone. Today, only 1 out of 10 survive a cardiac arrest with the current treatment and it is estimated to be the single largest cause of death worldwide.2 Neurescue aims for its device to help increase the survival rate for these patients.
“The FDA 510(k) clearance is an incredible milestone towards achieving our mission, saving the hearts and minds of emergency patients worldwide, giving us market authorization in the biggest medical device market in the world. Additionally, we have received FDA IDE approval to investigate a novel indication for the treatment of cardiac arrest,” said Habib Frost, M.D., founder and CEO of Neurescue. “We moved the bar forward for an often underserved, large group of patients. We’re excited about the tremendous potential of our device to improve outcomes for millions of patients and look forward to starting our U.S. clinical trial of the cardiac arrest indication.”
References
1 Cannon J. Hemorrhagic Shock. New England Journal Medicine. 2018; 378:370-379.
2 Virani et al. Heart Disease and Stroke Statistics—2020 Update: A Report from the American Heart Association. Circulation. 2020;141:e139–e596
The NEURESCUE device is the world’s first intelligent balloon catheter for aortic occlusion, an emergency technique that supercharges blood flow to the heart and brain within one minute from deployment. The catheter-based device is delivered via the femoral artery, temporarily inflating a soft balloon in the aorta to redirect blood flow towards the upper body. The procedure is performed to provide additional time to control blood loss and bridge patients to additional life-saving treatment options.
"The NEURESCUE device represents a significant advancement that could meaningfully improve the survival rates for emergency patients,” said Maham Rahimi, M.D., Ph.D., assistant professor of cardiovascular surgery at Weill Cornell School of Medicine. “The device gives patients suffering traumatic blood loss a longer window of time to receive appropriate medical interventions, which can directly translate to saved lives.”
Hemorrhage is a substantial global unmet need, with more than 60,000 deaths per year in the U.S., and an estimated 1.9 million deaths per year worldwide, 1.5 million of which result from trauma, such as car accidents.1 Cardiac arrest represents an even larger unmet need, with more than half a million deaths each year in the U.S. alone. Today, only 1 out of 10 survive a cardiac arrest with the current treatment and it is estimated to be the single largest cause of death worldwide.2 Neurescue aims for its device to help increase the survival rate for these patients.
“The FDA 510(k) clearance is an incredible milestone towards achieving our mission, saving the hearts and minds of emergency patients worldwide, giving us market authorization in the biggest medical device market in the world. Additionally, we have received FDA IDE approval to investigate a novel indication for the treatment of cardiac arrest,” said Habib Frost, M.D., founder and CEO of Neurescue. “We moved the bar forward for an often underserved, large group of patients. We’re excited about the tremendous potential of our device to improve outcomes for millions of patients and look forward to starting our U.S. clinical trial of the cardiac arrest indication.”
References
1 Cannon J. Hemorrhagic Shock. New England Journal Medicine. 2018; 378:370-379.
2 Virani et al. Heart Disease and Stroke Statistics—2020 Update: A Report from the American Heart Association. Circulation. 2020;141:e139–e596