Tom Fripp, Founder and Managing Director, Addition Design & Research01.30.19
Additive manufacturing (AM) processes (also known as 3D printing) have gained a great deal of traction across the medtech sector in the last decade. They offer companies a fabrication methodology capable of disrupting and improving upon the status quo, while also providing a means of differentiation within the market. On the other hand, the AM sector has suffered from inflated expectations, including from those within the medical device sector and its supply chain. What should not be in any doubt, however, is that AM can offer real business improvements that translate directly to a company’s bottom line. This is achieved by identifying real application opportunities where AM processes can be leveraged to offer true value, while recognizing the challenges involved. Perhaps more importantly, it also requires an understanding of when AM should not be pursued as a production process.
While the last statement might seem strange, this concept has been a challenging fundamental reality for many to grasp and impart on numerous projects and collaborations. On multiple occasions, companies have viewed AM as a panacea technology—rather than a tool in the toolbox—and are not maximizing its potential, but rather underutilizing it and often in the wrong places.
Prototyping Versus Production
To clarify, this article is focused on the application of AM processes as production techniques. This is not to minimize the most widespread application of 3D printing—prototyping and product development—where the technology set is growing and thriving as a tool that delivers fast and accurate physical iterations of products to arrive at optimal designs in terms of functionality and manufacturability. The continuous and consistent development of AM hardware capabilities and an increasing materials palette, however, along with the software ecosystem around them, are facilitating an escalation in production applications of AM across this industry.
This is where the greatest opportunities currently lie for companies in the medtech sector. Not only does AM offer real commercial opportunities for businesses developing innovative and complex products, but it can also have a profound and dramatic effect on the human condition in terms of improving the lives of the end users—the patients. This can be achieved directly at the point of need within clinical environments or communities, or indirectly as a manufacturing method for greatly improved medical devices. One example of direct applications is single-use surgical equipment where lead times can be reduced, equipment can be customized to the individual surgical procedure, waste can be minimized, and functionality of the instruments can be increased to achieve a better clinical outcome.
Currently, despite the direct approach dominating many headlines as surgeons and clinicians increasingly embrace the AM systems for customized devices at the point of need for patient-specific applications, it is the latter—the indirect approach—experiencing significant increases in volume, serial production where time and costs can be reduced in combination with a higher functioning product.
AM for Medtech
The medical sector is a colossal industry with many sub-segments. It is also an industry where money and politics often obscure the ultimate purpose of its existence—the health of humanity. The medical device industry (one subset of the medical sector) can also be further broken down into subsets based upon how a device is classified per its intended purpose, whether the device is anatomically invasive, and/or if the device contains active pharmaceutical ingredients for immediate delivery or if dispersed over time. This brief overview highlights the complexities of the genre, and this is even before taking into account the regulatory standards to which all medical devices are subjected. But where complexity exists, AM is often a good fit, supported by in-depth research, intelligent design approaches, and specifically tailored process and materials expertise. It is at the intersection of these disciplines where success with AM for production applications can be fostered.
Indeed, a key area for AM is the development of processes and materials that can satisfy the regulatory framework the medtech industry requires. As it is a business of risk reduction on a scale present in few other industries, changes are viewed in a critical light and benefits have to be substantial when it comes to solving problems. Although the barriers are high, the rewards are great. Further, as greater regulation across the AM sector is fueled by the aerospace industry’s adoption of AM, greater compliance for medtech will not be far behind.
Drilling Down
One classification of medical devices that shines a light on when, why, and how to maximize the potential of AM processes is medical implants—specifically orthopedic implants—which are subject to stringent regulation due to the critical nature of them being placed within the human body to fulfill the function of body parts that no longer work and cannot be repaired. When such a prognosis is delivered, it is invariably amid the suffering of ongoing physical pain that has a tremendous negative impact on an individual’s life and a long-term—and safe—resolution is the desired outcome.
In 2016, the U.S. Food & Drug Administration (FDA) said it had granted approval for 85 implant devices produced using AM—a significant proportion of which were for emergencies, not pre-planned surgeries. In 2018, this number continued to increase slowly in the pursuit of superior, cost-effective orthopedic implants produced using AM. An increasing number of medical device manufacturers—large and small—are utilizing an AM approach for the production of standard and customized orthopedic implants to achieve improved design and functionality.
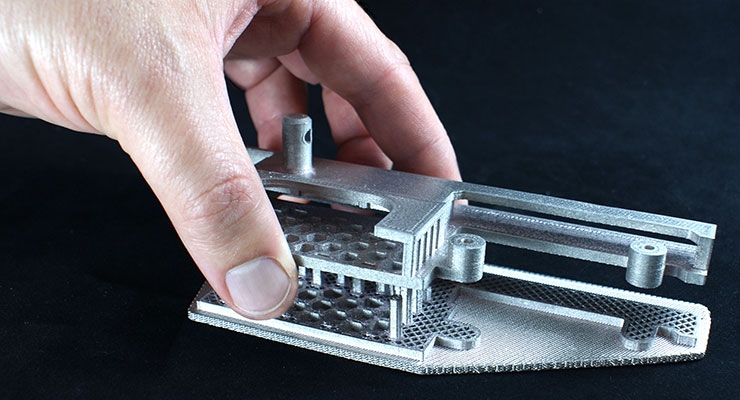
The dominant (but not exclusive) additive process in this specialized medical field is powder bed fusion (PBF), which encompasses selective laser melting (SLM), laser sintering (LS), and electron beam melting (EBM). This additive process (with the exception of EBM) can process both metal and polymer materials (metals, expectedly, dominate this specific application). It allows for the production of complex mesh structures for innovative implants that reduce costs and lead times compared with conventional manufacturing techniques for such products. The complexity involved in the production of an orthopedic implant is inherently linked to the ability to simulate bone structure, porosity, and a surface texture that produces high-friction and allows for bone ingrowth around the implant. This is where AM excels in a way no other single process has previously, which is why it presents as such an attractive prospect to medical device manufacturers.
Unsurprisingly, the medical device sector is a competitive market, with a dual focus on R&D and profit, much like its equally massive sister market of pharmaceuticals. For both, the emphasis is somewhat removed from the point of care, but companies are able to differentiate themselves by developing medical devices that facilitate improved—and longer lasting—treatments by clinicians that use them for their patients.
The highly regulated process of orthopedic implant production is not without challenges, of course. In terms of production with AM, parameters such as micron level accuracy and contamination risks are a couple of fundamental examples. As a result, it is not just AM hardware that has evolved to meet the demands of this application area but also validated quality assurance processes—for the powder material, the build process itself, and post processing requirements, such as sterilization.
It is also worth bearing in mind the adoption of AM is a two-way street, and the world of medicine and manufacturing has much to teach AM. For instance, good manufacturing practice is the mainstay of medical device manufacturing with in-process control checks and stringent regulations. AM systems designers will do well to think about how their technology can be better designed and built to foster and facilitate these practices without significant alteration to the established method of performing these tasks on current technology.
Increasing numbers of companies are working directly to develop standard additively manufactured orthopedic implants for different parts of the body. Each one demands specific anatomical research conducted in parallel with process and often material development, meaning it is not something that happens quickly in terms of getting the approval required for clinical use.
The orthopedic medical device industry is dominated by a small number of multi-billion dollar conglomerate organizations—notably Stryker, DePuy Synthes, and Smith & Nephew—all of which are now visibly conducting R&D with AM for a range of innovative devices. Stryker was among the earliest adopters. These multinationals, however, each work with extended supply chains and it is here that AM can have a real impact. Moreover, there is also evidence of an increase in smaller, specialist medical device manufacturers emerging over the last decade. In some cases, these companies have evolved their business around AM and are developing proprietary AM materials/processes for orthopedic implant development and production.
Moreover, there are some examples of NHS trusts developing their own manufacturing facilities to produce custom orthotic devices including Kent NHS Trust, which has developed and patented a method to produce orthotic insoles. Meanwhile, Manchester Metropolitan University is working with a U.K.-based firm to develop 3D-printed ocular prosthetics. In each case, the effects of AM adoption don’t end with the company as success also requires the supply chains for these collaborations to adjust to compensate for the new models of manufacture. On the smaller scale, surgeons and consultants are correctly identifying the possibilities of AM adoption and working with creative agencies who are able to maximize the benefits of AM use to develop one-off or small production runs of product for patients.
Conclusion
The opportunities for AM to more widely penetrate into the medical device sector are currently great. In certain circles, it might seem that it already has, but in reality, the existing applications of AM in these sectors is just a tiny fraction of what is possible. The key to unlocking these opportunities is a multi-faceted approach incorporating in-depth research with intelligent design for AM, specifically tailored for process and materials expertise, all within the framework of understanding medtech regulations. Working at the intersection of these disciplines fosters success with AM for medical production applications, and for companies within the supply chain, collaboration with companies that can bring this expertise and experience to the table is the best way of maximizing its potential.
Tom Fripp is the founder and managing director of Addition Design & Research. A former student of the Art and Design School of Sheffield Hallam University, Fripp received a First-Class Honours degree in industrial design followed by a distinction at master’s level, specializing in additive and digital manufacturing. In the following 12 years, he founded and grew an internationally recognized and multiple award-winning industrial design consultancy developing products for multinational clients as well as additive manufacturing technology, including the world’s first technique to produce 3D-printed facial prosthetics (a joint project with The Wellcome Trust) and a unique platinum silicone elastomer 3D-print technology. Addition Design & Research was formed in 2017 as a joint venture with the Chester Medical Solutions family of companies. The company pushes the boundaries of additive manufacturing technology through advanced design and research specifically for additive manufacture including the development of entirely new additive manufacturing technology as well as offering business focused 3D-print training. Fripp is passionate about any form of manufacture and is a Freeman of the Cutlers Company in Hallamshire.
While the last statement might seem strange, this concept has been a challenging fundamental reality for many to grasp and impart on numerous projects and collaborations. On multiple occasions, companies have viewed AM as a panacea technology—rather than a tool in the toolbox—and are not maximizing its potential, but rather underutilizing it and often in the wrong places.
Prototyping Versus Production
To clarify, this article is focused on the application of AM processes as production techniques. This is not to minimize the most widespread application of 3D printing—prototyping and product development—where the technology set is growing and thriving as a tool that delivers fast and accurate physical iterations of products to arrive at optimal designs in terms of functionality and manufacturability. The continuous and consistent development of AM hardware capabilities and an increasing materials palette, however, along with the software ecosystem around them, are facilitating an escalation in production applications of AM across this industry.
This is where the greatest opportunities currently lie for companies in the medtech sector. Not only does AM offer real commercial opportunities for businesses developing innovative and complex products, but it can also have a profound and dramatic effect on the human condition in terms of improving the lives of the end users—the patients. This can be achieved directly at the point of need within clinical environments or communities, or indirectly as a manufacturing method for greatly improved medical devices. One example of direct applications is single-use surgical equipment where lead times can be reduced, equipment can be customized to the individual surgical procedure, waste can be minimized, and functionality of the instruments can be increased to achieve a better clinical outcome.
Currently, despite the direct approach dominating many headlines as surgeons and clinicians increasingly embrace the AM systems for customized devices at the point of need for patient-specific applications, it is the latter—the indirect approach—experiencing significant increases in volume, serial production where time and costs can be reduced in combination with a higher functioning product.
AM for Medtech
The medical sector is a colossal industry with many sub-segments. It is also an industry where money and politics often obscure the ultimate purpose of its existence—the health of humanity. The medical device industry (one subset of the medical sector) can also be further broken down into subsets based upon how a device is classified per its intended purpose, whether the device is anatomically invasive, and/or if the device contains active pharmaceutical ingredients for immediate delivery or if dispersed over time. This brief overview highlights the complexities of the genre, and this is even before taking into account the regulatory standards to which all medical devices are subjected. But where complexity exists, AM is often a good fit, supported by in-depth research, intelligent design approaches, and specifically tailored process and materials expertise. It is at the intersection of these disciplines where success with AM for production applications can be fostered.
Indeed, a key area for AM is the development of processes and materials that can satisfy the regulatory framework the medtech industry requires. As it is a business of risk reduction on a scale present in few other industries, changes are viewed in a critical light and benefits have to be substantial when it comes to solving problems. Although the barriers are high, the rewards are great. Further, as greater regulation across the AM sector is fueled by the aerospace industry’s adoption of AM, greater compliance for medtech will not be far behind.
Drilling Down
One classification of medical devices that shines a light on when, why, and how to maximize the potential of AM processes is medical implants—specifically orthopedic implants—which are subject to stringent regulation due to the critical nature of them being placed within the human body to fulfill the function of body parts that no longer work and cannot be repaired. When such a prognosis is delivered, it is invariably amid the suffering of ongoing physical pain that has a tremendous negative impact on an individual’s life and a long-term—and safe—resolution is the desired outcome.
In 2016, the U.S. Food & Drug Administration (FDA) said it had granted approval for 85 implant devices produced using AM—a significant proportion of which were for emergencies, not pre-planned surgeries. In 2018, this number continued to increase slowly in the pursuit of superior, cost-effective orthopedic implants produced using AM. An increasing number of medical device manufacturers—large and small—are utilizing an AM approach for the production of standard and customized orthopedic implants to achieve improved design and functionality.
The dominant (but not exclusive) additive process in this specialized medical field is powder bed fusion (PBF), which encompasses selective laser melting (SLM), laser sintering (LS), and electron beam melting (EBM). This additive process (with the exception of EBM) can process both metal and polymer materials (metals, expectedly, dominate this specific application). It allows for the production of complex mesh structures for innovative implants that reduce costs and lead times compared with conventional manufacturing techniques for such products. The complexity involved in the production of an orthopedic implant is inherently linked to the ability to simulate bone structure, porosity, and a surface texture that produces high-friction and allows for bone ingrowth around the implant. This is where AM excels in a way no other single process has previously, which is why it presents as such an attractive prospect to medical device manufacturers.
Unsurprisingly, the medical device sector is a competitive market, with a dual focus on R&D and profit, much like its equally massive sister market of pharmaceuticals. For both, the emphasis is somewhat removed from the point of care, but companies are able to differentiate themselves by developing medical devices that facilitate improved—and longer lasting—treatments by clinicians that use them for their patients.
The highly regulated process of orthopedic implant production is not without challenges, of course. In terms of production with AM, parameters such as micron level accuracy and contamination risks are a couple of fundamental examples. As a result, it is not just AM hardware that has evolved to meet the demands of this application area but also validated quality assurance processes—for the powder material, the build process itself, and post processing requirements, such as sterilization.
It is also worth bearing in mind the adoption of AM is a two-way street, and the world of medicine and manufacturing has much to teach AM. For instance, good manufacturing practice is the mainstay of medical device manufacturing with in-process control checks and stringent regulations. AM systems designers will do well to think about how their technology can be better designed and built to foster and facilitate these practices without significant alteration to the established method of performing these tasks on current technology.
Increasing numbers of companies are working directly to develop standard additively manufactured orthopedic implants for different parts of the body. Each one demands specific anatomical research conducted in parallel with process and often material development, meaning it is not something that happens quickly in terms of getting the approval required for clinical use.
The orthopedic medical device industry is dominated by a small number of multi-billion dollar conglomerate organizations—notably Stryker, DePuy Synthes, and Smith & Nephew—all of which are now visibly conducting R&D with AM for a range of innovative devices. Stryker was among the earliest adopters. These multinationals, however, each work with extended supply chains and it is here that AM can have a real impact. Moreover, there is also evidence of an increase in smaller, specialist medical device manufacturers emerging over the last decade. In some cases, these companies have evolved their business around AM and are developing proprietary AM materials/processes for orthopedic implant development and production.
Moreover, there are some examples of NHS trusts developing their own manufacturing facilities to produce custom orthotic devices including Kent NHS Trust, which has developed and patented a method to produce orthotic insoles. Meanwhile, Manchester Metropolitan University is working with a U.K.-based firm to develop 3D-printed ocular prosthetics. In each case, the effects of AM adoption don’t end with the company as success also requires the supply chains for these collaborations to adjust to compensate for the new models of manufacture. On the smaller scale, surgeons and consultants are correctly identifying the possibilities of AM adoption and working with creative agencies who are able to maximize the benefits of AM use to develop one-off or small production runs of product for patients.
Conclusion
The opportunities for AM to more widely penetrate into the medical device sector are currently great. In certain circles, it might seem that it already has, but in reality, the existing applications of AM in these sectors is just a tiny fraction of what is possible. The key to unlocking these opportunities is a multi-faceted approach incorporating in-depth research with intelligent design for AM, specifically tailored for process and materials expertise, all within the framework of understanding medtech regulations. Working at the intersection of these disciplines fosters success with AM for medical production applications, and for companies within the supply chain, collaboration with companies that can bring this expertise and experience to the table is the best way of maximizing its potential.
Tom Fripp is the founder and managing director of Addition Design & Research. A former student of the Art and Design School of Sheffield Hallam University, Fripp received a First-Class Honours degree in industrial design followed by a distinction at master’s level, specializing in additive and digital manufacturing. In the following 12 years, he founded and grew an internationally recognized and multiple award-winning industrial design consultancy developing products for multinational clients as well as additive manufacturing technology, including the world’s first technique to produce 3D-printed facial prosthetics (a joint project with The Wellcome Trust) and a unique platinum silicone elastomer 3D-print technology. Addition Design & Research was formed in 2017 as a joint venture with the Chester Medical Solutions family of companies. The company pushes the boundaries of additive manufacturing technology through advanced design and research specifically for additive manufacture including the development of entirely new additive manufacturing technology as well as offering business focused 3D-print training. Fripp is passionate about any form of manufacture and is a Freeman of the Cutlers Company in Hallamshire.