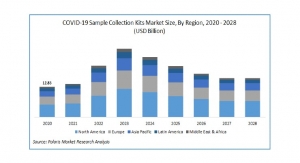
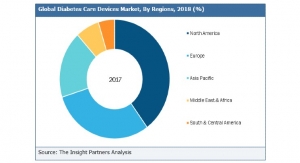
Kevin McIntosh, President of Summit Medical06.18.20
Closely monitoring early reports about the novel coronavirus, I quickly realized its potential to dramatically upend our lives— and livelihoods. Working with our client hospitals across the country, I knew doctors and nurses were enduring untenable delays in securing face shields and other critical personal protective equipment (PPE) due, in part, to components sourced outside of the United States.
As president of Summit Medical, a medical manufacturer based in St. Paul, Minn., I knew our capabilities — and established U.S. vendor relationships — would enable us to produce FDA compliant face shields with speed and efficiency. Our experience manufacturing specialty medical equipment and designing customized surgical instrument protection trays made pivoting to produce face shields a natural fit within our existing product lines and customer base.
I quickly convened our engineering team to determine how fast we could meet the growing need. The urgency was intensified as demand for Summit Medical’s elective surgical products declined with restrictions on medical procedures in states trying to preserve hospital space for the surge of COVID-19 patients. We not only wanted to protect healthcare workers, but we also wanted to protect our team members from layoffs.
Setting a Milestone Goal
Together, we established a goal to deliver more than one million FDA-compliant face shields to meet the critical need for PPE — not only for front-line health care workers, but also for returning business employees as the country reopens. We knew it was a demanding goal, but we were determined — and began working around the clock.
Our engineering team modified an open-source design from Maker Space at the University of Wisconsin-Madison to meet requirements for Summit Medical’s Face Shields to be registered and sold as a medical device. We conducted extensive research to ensure Summit Medical was providing the highest quality face shield.
Unlike masks, Summit Medical’s face shields protect all facial mucous membranes — including the eyes — from sprays, droplets, and fine aerosol contamination of the coronavirus. They enable visibility of the face and mouth, an important factor particularly for customer-facing businesses.
With our face shield prototype established, we tapped our network of suppliers to ensure our face shields would be sourced and manufactured entirely in the U.S. With production plans finalized and materials on-site, we cross-trained every staff member — including administrative staff — to make it possible to deliver face shields with both quality and speed.
In May, we achieved our first milestone to deliver over 1 million FDA-compliant face shields made entirely in the U.S. — in just eight weeks. Unlike others selling unregulated face shields during this limited period of relaxed standards, Summit Medical is an FDA-registered medical manufacturer and our quality and regulatory department has ensured that we have the capabilities to sustain and expand our production to meet the continued demand.
The effectiveness of face shields in health care demonstrates their utility to protect workers in other industries. As we continue to supply the health care community, we’re also offering a cost-effective way to protect employees returning to the office, sales floor, warehouse or industrial production floor as businesses reopen and adopt new COVID-19 infection-control protocols.
With our manufacturing processes in full swing in St. Paul, we decided to expand our face shield manufacturing with our sister companies under the Innovia Medical umbrella. We coordinated production at Eagle Labs in Rancho Cucamonga, Calif, initially shipping supplies from Minnesota until a local supply network was established while we provided assembly training.
Looking to extend our capabilities beyond the U.S., we collaborated with our sister companies to initiate face shield manufacturing at Network Medical and DTR Medical in the United Kingdom. We faced unique obstacles, including time differences and unique regulatory requirements, but we were able to begin production of Innovia Face Visors in less than two weeks. Now DTR Medical and Network Medical are supporting the National Health System (NHS) in the UK with over 1.3 million face shields.
To expand further, Summit Medical’s Quality and Regulatory team has received a CE certification allowing us to support the demand for face shields in Europe and beyond.
Kevin McIntosh is the president of Summit Medical, an Innovia Medical Company. He joined Summit Medical in 2010 and brought more than 33 years of technical and managerial experience in the development, manufacturing, and marketing of disposable and electromechanical medical devices. Formerly with SciMed Life Systems (now Boston Scientific) and Avecor Cardiovascular/Medtronic, McIntosh was instrumental in the design and development of a number of disposable instruments for cardiac surgery including the Magellan Platelet Separator technology and the Affinity bypass system product line (manufactured by Medtronic). He holds 24 issued patents and several pending patents.
As president of Summit Medical, a medical manufacturer based in St. Paul, Minn., I knew our capabilities — and established U.S. vendor relationships — would enable us to produce FDA compliant face shields with speed and efficiency. Our experience manufacturing specialty medical equipment and designing customized surgical instrument protection trays made pivoting to produce face shields a natural fit within our existing product lines and customer base.
I quickly convened our engineering team to determine how fast we could meet the growing need. The urgency was intensified as demand for Summit Medical’s elective surgical products declined with restrictions on medical procedures in states trying to preserve hospital space for the surge of COVID-19 patients. We not only wanted to protect healthcare workers, but we also wanted to protect our team members from layoffs.
Setting a Milestone Goal
Together, we established a goal to deliver more than one million FDA-compliant face shields to meet the critical need for PPE — not only for front-line health care workers, but also for returning business employees as the country reopens. We knew it was a demanding goal, but we were determined — and began working around the clock.
Our engineering team modified an open-source design from Maker Space at the University of Wisconsin-Madison to meet requirements for Summit Medical’s Face Shields to be registered and sold as a medical device. We conducted extensive research to ensure Summit Medical was providing the highest quality face shield.
Unlike masks, Summit Medical’s face shields protect all facial mucous membranes — including the eyes — from sprays, droplets, and fine aerosol contamination of the coronavirus. They enable visibility of the face and mouth, an important factor particularly for customer-facing businesses.
With our face shield prototype established, we tapped our network of suppliers to ensure our face shields would be sourced and manufactured entirely in the U.S. With production plans finalized and materials on-site, we cross-trained every staff member — including administrative staff — to make it possible to deliver face shields with both quality and speed.
In May, we achieved our first milestone to deliver over 1 million FDA-compliant face shields made entirely in the U.S. — in just eight weeks. Unlike others selling unregulated face shields during this limited period of relaxed standards, Summit Medical is an FDA-registered medical manufacturer and our quality and regulatory department has ensured that we have the capabilities to sustain and expand our production to meet the continued demand.
The effectiveness of face shields in health care demonstrates their utility to protect workers in other industries. As we continue to supply the health care community, we’re also offering a cost-effective way to protect employees returning to the office, sales floor, warehouse or industrial production floor as businesses reopen and adopt new COVID-19 infection-control protocols.
With our manufacturing processes in full swing in St. Paul, we decided to expand our face shield manufacturing with our sister companies under the Innovia Medical umbrella. We coordinated production at Eagle Labs in Rancho Cucamonga, Calif, initially shipping supplies from Minnesota until a local supply network was established while we provided assembly training.
Looking to extend our capabilities beyond the U.S., we collaborated with our sister companies to initiate face shield manufacturing at Network Medical and DTR Medical in the United Kingdom. We faced unique obstacles, including time differences and unique regulatory requirements, but we were able to begin production of Innovia Face Visors in less than two weeks. Now DTR Medical and Network Medical are supporting the National Health System (NHS) in the UK with over 1.3 million face shields.
To expand further, Summit Medical’s Quality and Regulatory team has received a CE certification allowing us to support the demand for face shields in Europe and beyond.
Kevin McIntosh is the president of Summit Medical, an Innovia Medical Company. He joined Summit Medical in 2010 and brought more than 33 years of technical and managerial experience in the development, manufacturing, and marketing of disposable and electromechanical medical devices. Formerly with SciMed Life Systems (now Boston Scientific) and Avecor Cardiovascular/Medtronic, McIntosh was instrumental in the design and development of a number of disposable instruments for cardiac surgery including the Magellan Platelet Separator technology and the Affinity bypass system product line (manufactured by Medtronic). He holds 24 issued patents and several pending patents.