Aaron Lieberman and Mark Hagerty, CCSVP09.19.19
We’re continually surprised by the many assumptions manufacturers make about how their surgical instruments will be cleaned, sterilized and protected. While their innovations are impressive, manufacturers consistently overestimate how their instruments will endure the real-world challenges of their valued customers.
While working with sterile processing and operating room staff across the country, we see increasingly delicate, complex — and expensive —instruments introduced into hospitals every day. The more specialized instruments entering the market — such as long, robotic, articulating instruments and intricate cameras —stretch the capabilities of most sterile processing departments. Basic assumptions like the facility having equipment that accommodates new instruments can undermine a manufacturer’s innovation and ultimately their reputation.
For example, are the facility’s sterilizers large enough to accommodate the dimensions of your new instrument? How will your instrument be positioned and protected during sterilization — and remain sterile during transport to and from the operating room?
These essential considerations tend to get the short-end of the innovation stick.
Misconceptions
The most common misconception is that facilities have the time and resources available to adhere to each manufacturer’s exact cleaning and sterilization instructions. Despite the best intentions, sterile processing departments are facing heightened demands for faster turnaround times to keep surgeries on schedule resulting in a higher likelihood of missing critical steps during cleaning and sterilization. These and many other assumptions are fast becoming an Achilles heel for surgical instrument manufacturers.
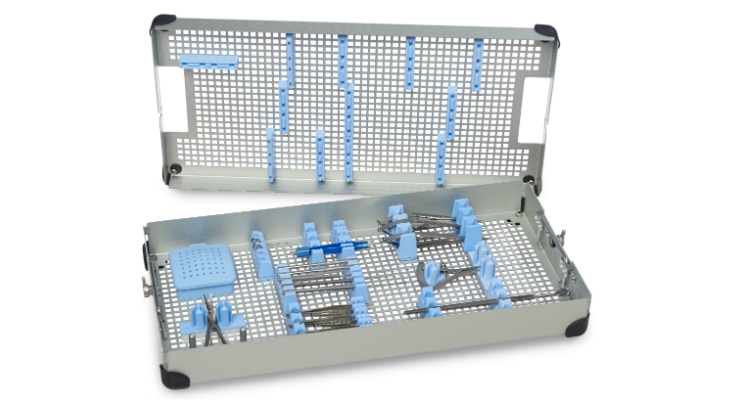
While manufacturers provide detailed cleaning and sterilization instructions, many do not pair their instrument with a custom tray to help ensure their instructions for use are properly followed. Without a custom tray, your instrument will likely be placed in a basic metal basket. Most metal baskets provide little to no protection and cannot ensure proper orientation for optimal sterilization.
This may seem obvious but our recommendation is to at minimum always pair your instrument with a protection tray. More specifically, we advise manufacturers to pair their instrument with a custom tray that is FDA validated ensuring their instrument is not only protected but optimally sterilized in various cycles.
Facilities Looking to Mitigate Risk
Facilities can no longer afford to assume additional financial risks for instrument repair and replacement. Because facilities are responsible for adhering to IFUs from hundreds of instruments, a custom tray supports their ability to accurately and efficiently manage processing while adhering to both your instructions and hospital accreditation standards.
To mitigate risk, many facilities prefer manufacturers to pair their instruments with an FDA validated tray. In fact, we’ve worked with several manufacturers who reached out to us after their customers requested their instruments be paired with a custom, validated tray.
The positioning of smaller instruments during sterile processing should also be carefully considered. For example, the phaco emulsifier used in cataract surgery—which is about the size of a highlighter—is typically placed horizontally in a metal basket for sterilization. However, a study published by the Journal of Hospital Infection demonstrated that when the phaco instrument is oriented vertically during sterilization, it is less likely to retain water. When placed horizontally (as it is done is most hospitals), it has a greater likelihood to retain water—and a greater potential to retain bacteria. Regulatory guidance during the manufacturing phase is invaluable as it’s difficult to stay up to date with best practices as new studies are being released every day.
Intelligent Instrument Tray Design
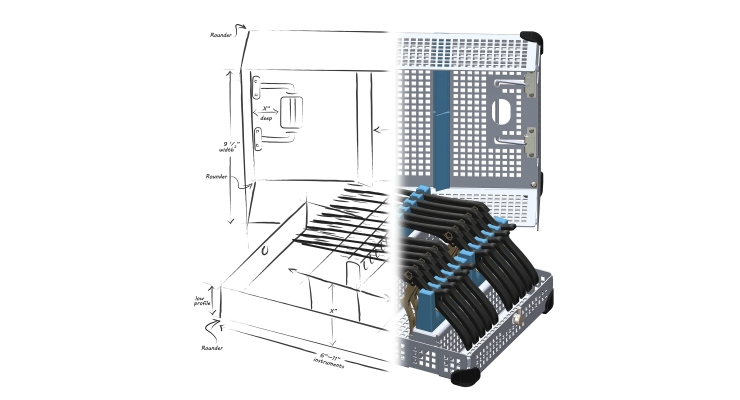
Though we’re not fond of the phrase “dummy proof,” we support its premise to design instrument trays that won’t require the end-user to spend an inordinate time to understand and adhere to the processing instructions.
In our work designing custom instrument protection trays, our engineers and designers often use the phrase “put some intelligence into it.” We recommend you ask smart, real-world questions to ensure you’re meeting the needs of your end users, such as:
We know there’s a tendency to presume best-case scenarios in the design process. Instrument manufacturers often have the advantage of a professional testing house or an internal department with the most advanced sterilization equipment — and the luxury of time to adhere to meticulous instructions. If you look at the design of your instrument in a vacuum, your IFU may seem clear and logical to you. But ask yourself: Will these instructions be quick and easy to follow for sterile processing technicians who are under pressure to quickly process dozens of instruments every day?
Intelligent custom tray design provides your instrument with a home that is thoughtfully designed to endure the rigors of everyday life—from sterile processing to the operating room and back—often several times a day. Pairing your instrument with a custom, FDA-validated tray offers immeasurable advantages for hospitals, patients—and ultimately your trusted brand reputation.
Aaron Lieberman (ALieberman@InnoviaMedical.com) is the group product manager for the InstruSafe Instrument Protection Line at Summit Medical, an Innovia Medical Company. His efforts have been instrumental to the multitude of product launches in his four years working with the InstruSafe product line. He holds a C.C.S.V.P certificate through IAHCSMM where he has been a member for four years.
Mark Hagerty (MHagerty@InnoviaMedical.com) is the InstruSafe business development specialist for Summit Medical, an Innovia Medical Company. He joined Summit Medical in 2014 as an engineer and after spending years designing products, he transitioned into product development where he manages the tray development process for all InstruSafe OEM clients. He holds a C.C.S.V.P certificate through IAHCSMM where he has been a member for four years.
While working with sterile processing and operating room staff across the country, we see increasingly delicate, complex — and expensive —instruments introduced into hospitals every day. The more specialized instruments entering the market — such as long, robotic, articulating instruments and intricate cameras —stretch the capabilities of most sterile processing departments. Basic assumptions like the facility having equipment that accommodates new instruments can undermine a manufacturer’s innovation and ultimately their reputation.
For example, are the facility’s sterilizers large enough to accommodate the dimensions of your new instrument? How will your instrument be positioned and protected during sterilization — and remain sterile during transport to and from the operating room?
These essential considerations tend to get the short-end of the innovation stick.
Misconceptions
The most common misconception is that facilities have the time and resources available to adhere to each manufacturer’s exact cleaning and sterilization instructions. Despite the best intentions, sterile processing departments are facing heightened demands for faster turnaround times to keep surgeries on schedule resulting in a higher likelihood of missing critical steps during cleaning and sterilization. These and many other assumptions are fast becoming an Achilles heel for surgical instrument manufacturers.
While manufacturers provide detailed cleaning and sterilization instructions, many do not pair their instrument with a custom tray to help ensure their instructions for use are properly followed. Without a custom tray, your instrument will likely be placed in a basic metal basket. Most metal baskets provide little to no protection and cannot ensure proper orientation for optimal sterilization.
This may seem obvious but our recommendation is to at minimum always pair your instrument with a protection tray. More specifically, we advise manufacturers to pair their instrument with a custom tray that is FDA validated ensuring their instrument is not only protected but optimally sterilized in various cycles.
Facilities Looking to Mitigate Risk
Facilities can no longer afford to assume additional financial risks for instrument repair and replacement. Because facilities are responsible for adhering to IFUs from hundreds of instruments, a custom tray supports their ability to accurately and efficiently manage processing while adhering to both your instructions and hospital accreditation standards.
To mitigate risk, many facilities prefer manufacturers to pair their instruments with an FDA validated tray. In fact, we’ve worked with several manufacturers who reached out to us after their customers requested their instruments be paired with a custom, validated tray.
The positioning of smaller instruments during sterile processing should also be carefully considered. For example, the phaco emulsifier used in cataract surgery—which is about the size of a highlighter—is typically placed horizontally in a metal basket for sterilization. However, a study published by the Journal of Hospital Infection demonstrated that when the phaco instrument is oriented vertically during sterilization, it is less likely to retain water. When placed horizontally (as it is done is most hospitals), it has a greater likelihood to retain water—and a greater potential to retain bacteria. Regulatory guidance during the manufacturing phase is invaluable as it’s difficult to stay up to date with best practices as new studies are being released every day.
Intelligent Instrument Tray Design
Though we’re not fond of the phrase “dummy proof,” we support its premise to design instrument trays that won’t require the end-user to spend an inordinate time to understand and adhere to the processing instructions.
In our work designing custom instrument protection trays, our engineers and designers often use the phrase “put some intelligence into it.” We recommend you ask smart, real-world questions to ensure you’re meeting the needs of your end users, such as:
- How will the instrument be properly loaded and stabilized during the sterilization process?
- What kind of creative barriers, angles and imagery could be incorporated in the tray to ensure the instrument is accurately loaded?
- How will the position of the instrument in the tray allow optimum access to sterilant?
- How will each part of the instrument be accounted for and easily secured?
- How will the instrument remain sterile in transport from sterile processing to the operating room?
We know there’s a tendency to presume best-case scenarios in the design process. Instrument manufacturers often have the advantage of a professional testing house or an internal department with the most advanced sterilization equipment — and the luxury of time to adhere to meticulous instructions. If you look at the design of your instrument in a vacuum, your IFU may seem clear and logical to you. But ask yourself: Will these instructions be quick and easy to follow for sterile processing technicians who are under pressure to quickly process dozens of instruments every day?
Intelligent custom tray design provides your instrument with a home that is thoughtfully designed to endure the rigors of everyday life—from sterile processing to the operating room and back—often several times a day. Pairing your instrument with a custom, FDA-validated tray offers immeasurable advantages for hospitals, patients—and ultimately your trusted brand reputation.
Aaron Lieberman (ALieberman@InnoviaMedical.com) is the group product manager for the InstruSafe Instrument Protection Line at Summit Medical, an Innovia Medical Company. His efforts have been instrumental to the multitude of product launches in his four years working with the InstruSafe product line. He holds a C.C.S.V.P certificate through IAHCSMM where he has been a member for four years.
Mark Hagerty (MHagerty@InnoviaMedical.com) is the InstruSafe business development specialist for Summit Medical, an Innovia Medical Company. He joined Summit Medical in 2014 as an engineer and after spending years designing products, he transitioned into product development where he manages the tray development process for all InstruSafe OEM clients. He holds a C.C.S.V.P certificate through IAHCSMM where he has been a member for four years.