Brian Semcer, President, MICRO09.29.20
Over the last 75 years, medical device manufacturing has experienced a dramatic evolution. Technological advances, including software, nanotechnology, and sensor technology, have significantly impacted the industry, and healthcare delivery, in countless ways. MICRO is celebrating its 75th anniversary in 2020 and today, we look back on a completely changed landscape, where surgery is more efficient and safer than ever before and outcomes for patients have greatly improved.

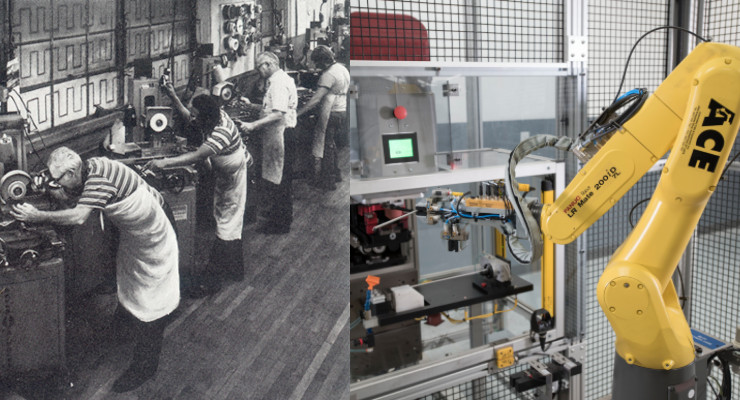
Microstamping on the manufacturing floor in 1945
Engineering techniques, materials, and manufacturing processes have evolved and improved over time in parallel, largely enabled by technology. Today, medical device manufacturers are using robotics, 3D printing, and materials science to produce new and improved parts and products that meet market, customer, and patient needs. The complexity and sophistication of the outputs have grown exponentially, as minimally invasive instruments and computer-enabled surgical tools become more automated, reproduceable, and portable.

Frank Semcer Sr. at Microstamping
Our company was founded in 1945 and initially focused on manufacturing high-precision metal stampings. What started as a highly specialized focus over time evolved to become a full-service contract manufacturing organization (CMO) with expertise in prototyping and validation for full-scale production. Our modern manufacturing techniques and innovative engineering solutions help ensure consistent development and delivery of high-quality products for the customer.
Outsourcing now plays a critical role in medical device manufacturing, as contract manufacturers find new ways to produce parts and products consistently and cost-effectively. As the medical device marketplace continues to expand, CMOs need to stay ahead of the curve with quality and compliance systems and an understanding of global regulations and industry best practices. The growth in new products, technologies, systems, and tools in medical device manufacturing, coupled with an expansion of the market globally, has been met with an increasingly complex regulatory landscape. Regulations are now market-specific and there can be enormous variability depending on the market or country.
Additionally, regulatory standards have become more complex as new technologies enter the commercial market. For example, certain biologics and tissue-based products may now be regulated as medical devices; pharmaceutical coatings applied to medical devices have given rise to combination products; and implantable electronic devices must comply with new regulatory requirements and guidance. With a growing demand for off-the-shelf sterile products, manufacturers also need to ensure they comply with standards for cleanroom and controlled environments. Product testing and production controls remain a primary focus for contract manufacturers, along with safety and quality control standards, and more resources may be needed to ensure safety standards and requirements for infection control are met.

Very delicate, high-volume components are best handled by robotic cells.
CMOs have always had to be nimble and shift gears quickly to address changing market needs. In addition to an evolution in technology, manufacturing processes, and regulations, our customers face greater time and financial pressures than ever before. Increased competition and a customer-centric bottom line market mean that best-in-class manufacturing processes are needed to optimize production cycle times and meet cost targets. A CMO partner that can help the customer get their products to market on time and at cost, and meet quality requirements, is invaluable. Innovation is the hallmark of our business and we continually seek ways to improve and reinvest in technology, equipment, state-of-the-art facilities, and training to create new and better solutions for customers and, ultimately, patients. Technology investments are essential and will help CMOs prepare for future growth and stay competitive. This includes accelerating new areas of data and analytics such as machine learning and predictive quality and maintenance.

John Semcer, toolmaker and older brother of MICRO Founder Frank Semcer Sr.
Since our earliest days as a company, our philosophy has always remained the same—bring technical expertise and excellence in customer service to every client. We must evolve with the technology to support our customers. To be successful for 75 years requires an unrelenting focus on the customer, in addition to engineering expertise and a deep knowledge base. Exceeding the customer’s expectations has been at the core of our business since its very beginning. Technology will continue to expand the market and shape our business. With innovation, quality, and safety as the cornerstones in manufacturing and engineering, we can and will continue to meet customer needs and adapt to new market demands over time.

Microstamping on the manufacturing floor in 1945
Engineering techniques, materials, and manufacturing processes have evolved and improved over time in parallel, largely enabled by technology. Today, medical device manufacturers are using robotics, 3D printing, and materials science to produce new and improved parts and products that meet market, customer, and patient needs. The complexity and sophistication of the outputs have grown exponentially, as minimally invasive instruments and computer-enabled surgical tools become more automated, reproduceable, and portable.

Frank Semcer Sr. at Microstamping
Outsourcing now plays a critical role in medical device manufacturing, as contract manufacturers find new ways to produce parts and products consistently and cost-effectively. As the medical device marketplace continues to expand, CMOs need to stay ahead of the curve with quality and compliance systems and an understanding of global regulations and industry best practices. The growth in new products, technologies, systems, and tools in medical device manufacturing, coupled with an expansion of the market globally, has been met with an increasingly complex regulatory landscape. Regulations are now market-specific and there can be enormous variability depending on the market or country.
Additionally, regulatory standards have become more complex as new technologies enter the commercial market. For example, certain biologics and tissue-based products may now be regulated as medical devices; pharmaceutical coatings applied to medical devices have given rise to combination products; and implantable electronic devices must comply with new regulatory requirements and guidance. With a growing demand for off-the-shelf sterile products, manufacturers also need to ensure they comply with standards for cleanroom and controlled environments. Product testing and production controls remain a primary focus for contract manufacturers, along with safety and quality control standards, and more resources may be needed to ensure safety standards and requirements for infection control are met.

Very delicate, high-volume components are best handled by robotic cells.
CMOs have always had to be nimble and shift gears quickly to address changing market needs. In addition to an evolution in technology, manufacturing processes, and regulations, our customers face greater time and financial pressures than ever before. Increased competition and a customer-centric bottom line market mean that best-in-class manufacturing processes are needed to optimize production cycle times and meet cost targets. A CMO partner that can help the customer get their products to market on time and at cost, and meet quality requirements, is invaluable. Innovation is the hallmark of our business and we continually seek ways to improve and reinvest in technology, equipment, state-of-the-art facilities, and training to create new and better solutions for customers and, ultimately, patients. Technology investments are essential and will help CMOs prepare for future growth and stay competitive. This includes accelerating new areas of data and analytics such as machine learning and predictive quality and maintenance.

John Semcer, toolmaker and older brother of MICRO Founder Frank Semcer Sr.