Abbott09.21.20
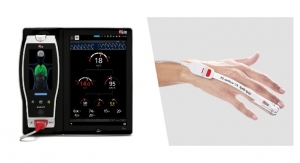
Abbott has received a CE Mark for its fourth-generation MitraClip Transcatheter Mitral Valve Repair System, a minimally invasive mitral valve repair device. The device is now approved for use in Europe and other countries that recognize CE Mark as a non-surgical option for the treatment of mitral regurgitation (MR), or a leaky heart valve. The device is already approved for use in the U.S.
The MitraClip G4 system provides physicians in Europe with enhancements to MitraClip's clip-based technology, building upon the device's proven delivery system. In addition to offering advanced steering during implantation, the new delivery system offers four clip sizes, including two wider clips, for doctors to have a greater variety of treatment options that can be tailored to a patients' unique mitral valve anatomy.1 The newest-generation device also offers independently controlled grippers, if needed, that allow physicians to grasp one or both mitral valve leaflets at a time during the MitraClip procedure.
Mitral Regurgitation
MR is one of the most common heart conditions, affecting one in 10 adults age 75 and older.2,3 Patients with this progressive condition have a mitral valve that does not close completely, allowing blood to flow backward into the left atrium of the heart instead of forwards (out of the heart) and to the rest of the body. While medication can help people manage the symptoms of MR, it does not treat the leaky valve itself. Prior to MitraClip, open-heart surgery was the standard treatment for MR, however, not all patients are eligible or appropriate for open-heart surgery due to the potential risk of complications stemming from comorbidities, advanced age, or other issues.
MitraClip is a minimally invasive transcatheter mitral valve repair (TMVr) therapy that can be a life-saving treatment option for select patients with primary or secondary MR.4 The small clip-based device is delivered to the heart through a vein in the leg and clips portions of the leaflets, or flaps, of the mitral valve together to reduce the backflow of blood. Once in place, MitraClip restores the proper functioning of the mitral valve and the heart's ability to pump oxygenated blood more efficiently.
"Despite being consistently recognized as a problem in patients around the world, MR cannot be treated through the conventional method of open-heart mitral valve surgery in more than half of the people who have this condition," said Ralph Stephan Von Bardeleben, head of the Heart Valve Center Mainz, Universitätsmedizin Mainz, Germany, who treated the first MitraClip G4 patients in the EU. "The newest MitraClip therapy offers physicians a reliable option when surgical treatment of MR isn't possible or appropriate, and MitraClip G4's enhancements allow further customization of the therapy to tailor treatment to individual patient needs."
Clinical Studies of MitraClip
MitraClip is backed by more than 16 years of clinical experience with proven safety, survival and durable clinical outcomes. Data presented at PCR e-Course this year from a real-world clinical study of over 1,000 MitraClip patients showed a high implant success rate (99%) and demonstrated MR reduction to the level of none or trace in patients with either primary MR (to ≤1+ in 87.1%) or secondary MR (to ≤1+ in 90.1%) at 30 days. This recent data confirms MitraClip's best-in-class MR reduction to date1 and adds to a body of evidence that demonstrates significant impact for patients, including improved clinical outcomes and quality of life.
"An enduring measure of our mission to help people live better lives through better health is our success in advancing new standards of care for the treatment of structural heart diseases," said Michael Dale, senior vice president of Abbott's structural heart business. "This CE Mark, along with other recent approvals and advancements for our MitraClip device, underscores the need for MitraClip's innovative therapy – which has become a preferred choice for the treatment of mitral regurgitation around the world."
References:
1 Rottbauer W. D. Contemporary Clinical Outcomes with MitraClip™ (NTR/XTR) System: Core-lab Echo Results from +1000 Patient the Global EXPAND Study. Data presented at PCR 2020.
2 Lloyd-Jones D, Adams RJ, Brown TM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46-e215.
3 Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368(9540):1005-1011.
4 Primary MR is caused by an anatomic defect of one or more of the structures of the mitral valve of the heart, while secondary MR occurs in patients with coronary disease, wherein the damage from the disease impairs the performance of a normal mitral valve.
The MitraClip G4 system provides physicians in Europe with enhancements to MitraClip's clip-based technology, building upon the device's proven delivery system. In addition to offering advanced steering during implantation, the new delivery system offers four clip sizes, including two wider clips, for doctors to have a greater variety of treatment options that can be tailored to a patients' unique mitral valve anatomy.1 The newest-generation device also offers independently controlled grippers, if needed, that allow physicians to grasp one or both mitral valve leaflets at a time during the MitraClip procedure.
Mitral Regurgitation
MR is one of the most common heart conditions, affecting one in 10 adults age 75 and older.2,3 Patients with this progressive condition have a mitral valve that does not close completely, allowing blood to flow backward into the left atrium of the heart instead of forwards (out of the heart) and to the rest of the body. While medication can help people manage the symptoms of MR, it does not treat the leaky valve itself. Prior to MitraClip, open-heart surgery was the standard treatment for MR, however, not all patients are eligible or appropriate for open-heart surgery due to the potential risk of complications stemming from comorbidities, advanced age, or other issues.
MitraClip is a minimally invasive transcatheter mitral valve repair (TMVr) therapy that can be a life-saving treatment option for select patients with primary or secondary MR.4 The small clip-based device is delivered to the heart through a vein in the leg and clips portions of the leaflets, or flaps, of the mitral valve together to reduce the backflow of blood. Once in place, MitraClip restores the proper functioning of the mitral valve and the heart's ability to pump oxygenated blood more efficiently.
"Despite being consistently recognized as a problem in patients around the world, MR cannot be treated through the conventional method of open-heart mitral valve surgery in more than half of the people who have this condition," said Ralph Stephan Von Bardeleben, head of the Heart Valve Center Mainz, Universitätsmedizin Mainz, Germany, who treated the first MitraClip G4 patients in the EU. "The newest MitraClip therapy offers physicians a reliable option when surgical treatment of MR isn't possible or appropriate, and MitraClip G4's enhancements allow further customization of the therapy to tailor treatment to individual patient needs."
Clinical Studies of MitraClip
MitraClip is backed by more than 16 years of clinical experience with proven safety, survival and durable clinical outcomes. Data presented at PCR e-Course this year from a real-world clinical study of over 1,000 MitraClip patients showed a high implant success rate (99%) and demonstrated MR reduction to the level of none or trace in patients with either primary MR (to ≤1+ in 87.1%) or secondary MR (to ≤1+ in 90.1%) at 30 days. This recent data confirms MitraClip's best-in-class MR reduction to date1 and adds to a body of evidence that demonstrates significant impact for patients, including improved clinical outcomes and quality of life.
"An enduring measure of our mission to help people live better lives through better health is our success in advancing new standards of care for the treatment of structural heart diseases," said Michael Dale, senior vice president of Abbott's structural heart business. "This CE Mark, along with other recent approvals and advancements for our MitraClip device, underscores the need for MitraClip's innovative therapy – which has become a preferred choice for the treatment of mitral regurgitation around the world."
References:
1 Rottbauer W. D. Contemporary Clinical Outcomes with MitraClip™ (NTR/XTR) System: Core-lab Echo Results from +1000 Patient the Global EXPAND Study. Data presented at PCR 2020.
2 Lloyd-Jones D, Adams RJ, Brown TM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46-e215.
3 Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368(9540):1005-1011.
4 Primary MR is caused by an anatomic defect of one or more of the structures of the mitral valve of the heart, while secondary MR occurs in patients with coronary disease, wherein the damage from the disease impairs the performance of a normal mitral valve.