GlobeNewswire09.01.20
T2 Biosystems Inc., a firm specializing in the rapid detection of sepsis-causing pathogens, announced the U.S. Food and Drug Administration (FDA) has issued Emergency Use Authorization (EUA) for its COVID-19 molecular diagnostic test, the T2SARS-CoV-2 Panel.
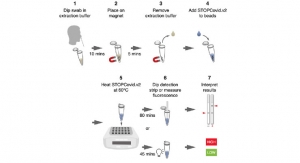
The T2SARS-CoV-2 Panel is a molecular diagnostic test that detects SARS-CoV-2, the virus responsible for COVID-19 infections, and provides results in under two hours utilizing a nasopharyngeal swab sample. The test runs on the Company’s FDA-cleared and fully-automated T2Dx Instrument, which is capable of performing seven tests simultaneously. Clinical testing on both positive and negative patient samples demonstrated a sensitivity of 95 percent and specificity of 100 percent.
“We believe the Emergency Use Authorization for our COVID-19 molecular diagnostic test will lead to greater adoption of our technology by U.S. hospitals, and we are expanding manufacturing capacity to meet the anticipated demand,” said John Sperzel, President and Chief Executive Officer of T2 Biosystems. “As critically-ill COVID-19 patients are susceptible to bacterial or fungal co-infections and secondary infections that can lead to sepsis, we believe our platform can be an effective tool to identify acute COVID-19 infections, and optimize outcomes for hospitalized patients under intensive care.”
The T2Dx Instrument can also run the company’s FDA-cleared T2Bacteria Panel and T2Candida Panel. These panels are the only FDA-cleared assays for the detection of sepsis-causing bacterial and fungal pathogens directly from whole blood in three to five hours, without the need to wait days for blood culture results. By providing quicker results, the panels enable clinicians to target therapy faster for their patients suspected of sepsis, often before the second dose of antimicrobial is administered, leading to better patient outcomes, improved antimicrobial stewardship, and reductions in length of stay in the hospital.
The T2SARS-CoV-2 Panel is a molecular diagnostic test that detects SARS-CoV-2, the virus responsible for COVID-19 infections, and provides results in under two hours utilizing a nasopharyngeal swab sample. The test runs on the Company’s FDA-cleared and fully-automated T2Dx Instrument, which is capable of performing seven tests simultaneously. Clinical testing on both positive and negative patient samples demonstrated a sensitivity of 95 percent and specificity of 100 percent.
“We believe the Emergency Use Authorization for our COVID-19 molecular diagnostic test will lead to greater adoption of our technology by U.S. hospitals, and we are expanding manufacturing capacity to meet the anticipated demand,” said John Sperzel, President and Chief Executive Officer of T2 Biosystems. “As critically-ill COVID-19 patients are susceptible to bacterial or fungal co-infections and secondary infections that can lead to sepsis, we believe our platform can be an effective tool to identify acute COVID-19 infections, and optimize outcomes for hospitalized patients under intensive care.”
The T2Dx Instrument can also run the company’s FDA-cleared T2Bacteria Panel and T2Candida Panel. These panels are the only FDA-cleared assays for the detection of sepsis-causing bacterial and fungal pathogens directly from whole blood in three to five hours, without the need to wait days for blood culture results. By providing quicker results, the panels enable clinicians to target therapy faster for their patients suspected of sepsis, often before the second dose of antimicrobial is administered, leading to better patient outcomes, improved antimicrobial stewardship, and reductions in length of stay in the hospital.