Christine Scifert and Dawn Norman, M.S., MRC-X LLC10.01.20
This column will provide information related to U.S. Food and Drug Administration (FDA) Emergency Use Authorizations (EUAs) granted due to the global COVID-19 pandemic. The current public health threat has justified implementing emergency use authorization for diagnostic, non-diagnostic, and therapeutic medical devices used to detect and/or diagnose COVID-19. Such authorizations have helped make important medical products available more quickly, particularly during shortages of approved and available alternative devices such as personal respiratory protective gear and in-vitro diagnostics.
COVID-19 is not the first public health emergency where EUAs have been issued but it is by far the most extensive. EUAs were also issued for Zika Virus, the 2014 Ebola Virus, and the 2013 H7N9 Influenza, allowing alternative assay testing for faster detection. FDA can use its EUA authority to allow the use of unapproved medical devices or unapproved uses of approved medical devices to diagnose and treat COVID-19. These authorizations are not subject to specific regulatory requirements during the public health emergency but there are restrictions to the use and labeling requirements imposed on these devices. The next section of this column details the device types that have received this authorization.
Scope of Devices for EUA
Medical device types in which EUAs may apply may be found in one of the following categories:
Enforcement Discretion—Product-Specific and Quality System
Medical device classification and potential risk determines the regulatory pathway and requirements for devices. For example, Class I devices such as face shields have a more broad set of requirements for EUA as opposed to more specific guidance and requirements for higher risk and class devices. Guidance for these lower risk devices also provides allowances where they are not subject to the following regulatory requirements in some cases:
Additional labeling requirements apply to the manufacturers and distributors of these authorized products; for example, face shields must identify the device as single-use or for use by a single user and include instructions for cleaning and/or disinfection if applicable. Advertising or promotional materials cannot suggest the product is safe or effective for the prevention or treatment of patients during the COVID-19 pandemic and shall clearly identify that the product has not been FDA approved and is only authorized for use only during declaration of the EUA.
In contrast with exemptions from regulatory requirements allowable for Class I devices, Class II devices are not exempt from 21 CFR 806 (reports of corrections or removals) or 21 CFR 820 (Quality System Regulation requirements).
The exemptions for EUA devices will again be enforced after the public health emergency is over. It is currently impossible to determine when the health emergency will conclude. Therefore, plans should be made to address each of the previously mentioned excluded requirements if there is a desire to continue marketing the device long-term.
Timelines and Long-Term Planning
Once the exemptions for EUA devices end, manufacturers will have to comply with FDA regulations. Depending on the device classification, it will be important to have a plan in place for the following:
Conclusion
Manufacturers must comply with the current EUA requirements before distributing products. In addition, these manufacturers are subject to FDA enforcement discretion due to EUAs and if they want to continue marketing these devices, they must be prepared to comply to all regulations and requirements. These requirements can include registration and listing, quality system regulation, reporting of corrections and removals, or UDI requirements when the COVID-19 public health emergency concludes. These considerations pertain only to the U.S. market and FDA.
References
Christine Scifert is co-founder of MRC-X LLC (previously Memphis Regulatory Consulting LLC), which offers medical device regulatory, quality and clinical services. Prior to MRC Global, Scifert spent 11 years as a consultant with MRC-X LLC and Memphis Regulatory Consulting and nine years at Medtronic Spinal and Biologics directing the regulatory department of 22 people. As senior director, she set regulatory strategy, oversaw global submissions and developed a design control process. Prior to Medtronic, Scifert performed evaluations of injury mechanisms associated with automobile collisions, slip and falls, and sport/recreation accidents. Scifert earned a bachelor of science degree in physics from Hamline University and a masters of science degree in biomedical engineering from University of Iowa. She also completed a master’s degree in engineering management from Christian Brothers University. Scifert is also an adjunct professor at Christian Brothers University teaching regulatory affairs and presents nationally as a regulatory expert at various industry events.
Prior to MRC Global, Dawn Norman spent 15 years in the medical device industry working with venture capital backed startups and larger companies alike. She focused on regulatory strategy and submissions, along with clinical study design and study execution. Norman was directly involved with early development and market entry for leading-edge medical innovation including magnetic navigation devices for neurosurgical, neurovascular and cardiac indications, high intensity ultrasound for cardiac ablation, recombinant proteins for bone fusion, orthopedic trauma, infusion pumps, and advanced imaging technologies. Norman earned a bachelor of arts degree in biological sciences and chemistry, and a masters of science degree in biomedical sciences from Southern Illinois University at Edwardsville. She is also a certified ISO 13485 lead auditor. Norman is an industry expert and presents at various trade shows/conferences.
COVID-19 is not the first public health emergency where EUAs have been issued but it is by far the most extensive. EUAs were also issued for Zika Virus, the 2014 Ebola Virus, and the 2013 H7N9 Influenza, allowing alternative assay testing for faster detection. FDA can use its EUA authority to allow the use of unapproved medical devices or unapproved uses of approved medical devices to diagnose and treat COVID-19. These authorizations are not subject to specific regulatory requirements during the public health emergency but there are restrictions to the use and labeling requirements imposed on these devices. The next section of this column details the device types that have received this authorization.
Scope of Devices for EUA
Medical device types in which EUAs may apply may be found in one of the following categories:
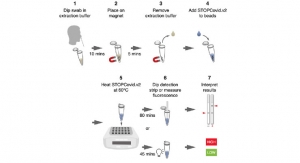
- In-vitro diagnostic products, high complexity molecular-based tests, and SARS-CoV-2 antibody tests to detect parts of the virus, antibodies, or the biomarkers related to COVID-19-related inflammation
- Personal protective equipment, such as protective clothing, surgical and non-surgical gowns, helmets, gloves, face shields, intubation shields, goggles, respirators, or other equipment designed to protect people from the spread of COVID-19
- Ventilators and respiratory assistance devices, and devices used for their decontamination to control and support patient breathing and allowing for reuse of certain respirators in short supply
- Blood purification and continuous renal replacement therapy and hemodialysis devices to help reduce small active proteins in the bloodstream, which are typically elevated during infections and lead to more severe reactions. These products help to filter and clean blood when the kidneys are not functioning normally
- Infusion pumps, and remote or wearable patient monitoring devices to help increase the availability of devices delivering controlled levels of fluids and medications, or for monitoring and treating patients to help reduce viral exposure to healthcare providers
Enforcement Discretion—Product-Specific and Quality System
Medical device classification and potential risk determines the regulatory pathway and requirements for devices. For example, Class I devices such as face shields have a more broad set of requirements for EUA as opposed to more specific guidance and requirements for higher risk and class devices. Guidance for these lower risk devices also provides allowances where they are not subject to the following regulatory requirements in some cases:
- Registration and Listing requirements in 21 CFR 807
- Quality System Regulation requirements in 21 CFR 820
- Reports of corrections and removals in 21 CFR Part 806
- Unique Device Identification requirements in 21 CFR Part 830 and 21 CFR 801.20
Additional labeling requirements apply to the manufacturers and distributors of these authorized products; for example, face shields must identify the device as single-use or for use by a single user and include instructions for cleaning and/or disinfection if applicable. Advertising or promotional materials cannot suggest the product is safe or effective for the prevention or treatment of patients during the COVID-19 pandemic and shall clearly identify that the product has not been FDA approved and is only authorized for use only during declaration of the EUA.
In contrast with exemptions from regulatory requirements allowable for Class I devices, Class II devices are not exempt from 21 CFR 806 (reports of corrections or removals) or 21 CFR 820 (Quality System Regulation requirements).
The exemptions for EUA devices will again be enforced after the public health emergency is over. It is currently impossible to determine when the health emergency will conclude. Therefore, plans should be made to address each of the previously mentioned excluded requirements if there is a desire to continue marketing the device long-term.
Timelines and Long-Term Planning
Once the exemptions for EUA devices end, manufacturers will have to comply with FDA regulations. Depending on the device classification, it will be important to have a plan in place for the following:
- Registration and Listing requirements in 21 CFR 807, where manufacturers are required to register their facility and list each product sold. This establishment registration fee is $5,236 for Fiscal Year 2020.
- Quality System Regulation requirements in 21 CFR 820, where the quality system regulation governs the methods used in, and the facilities and controls used for the design, manufacture, packaging, labeling, storage, installation, and servicing of all finished devices intended for human use.
- Reports of corrections and removals in 21 CFR 806, where manufacturers are required to promptly report certain actions concerning device corrections and removals (recalls), and to maintain records of corrections and removals.
- Unique Device Identification requirements in 21 CFR Part 830 and 21 CFR 801.20, where the label of every medical device must bear a unique device identifier (UDI) registered with FDA through the Global Unique Device Identification Database (GUDID).
Conclusion
Manufacturers must comply with the current EUA requirements before distributing products. In addition, these manufacturers are subject to FDA enforcement discretion due to EUAs and if they want to continue marketing these devices, they must be prepared to comply to all regulations and requirements. These requirements can include registration and listing, quality system regulation, reporting of corrections and removals, or UDI requirements when the COVID-19 public health emergency concludes. These considerations pertain only to the U.S. market and FDA.
References
- Simpson J.P, et al., Measurement of airborne particle exposure during simulated tracheal intubation using various proposed aerosol containment devices during the COVID-19 pandemic, Anesthesia, 19 June 2020, 1-9.
- Begley J.I. et al., The Aerosol box for intubation in COVID-19 patients: an in-situ simulation crossover study, Anesthesia, August 20202. 75 (8), 1014-1021.
Christine Scifert is co-founder of MRC-X LLC (previously Memphis Regulatory Consulting LLC), which offers medical device regulatory, quality and clinical services. Prior to MRC Global, Scifert spent 11 years as a consultant with MRC-X LLC and Memphis Regulatory Consulting and nine years at Medtronic Spinal and Biologics directing the regulatory department of 22 people. As senior director, she set regulatory strategy, oversaw global submissions and developed a design control process. Prior to Medtronic, Scifert performed evaluations of injury mechanisms associated with automobile collisions, slip and falls, and sport/recreation accidents. Scifert earned a bachelor of science degree in physics from Hamline University and a masters of science degree in biomedical engineering from University of Iowa. She also completed a master’s degree in engineering management from Christian Brothers University. Scifert is also an adjunct professor at Christian Brothers University teaching regulatory affairs and presents nationally as a regulatory expert at various industry events.
Prior to MRC Global, Dawn Norman spent 15 years in the medical device industry working with venture capital backed startups and larger companies alike. She focused on regulatory strategy and submissions, along with clinical study design and study execution. Norman was directly involved with early development and market entry for leading-edge medical innovation including magnetic navigation devices for neurosurgical, neurovascular and cardiac indications, high intensity ultrasound for cardiac ablation, recombinant proteins for bone fusion, orthopedic trauma, infusion pumps, and advanced imaging technologies. Norman earned a bachelor of arts degree in biological sciences and chemistry, and a masters of science degree in biomedical sciences from Southern Illinois University at Edwardsville. She is also a certified ISO 13485 lead auditor. Norman is an industry expert and presents at various trade shows/conferences.