Videos
Cepheid's Combo COVID-19, Flu A/B, RSV Test Earns EUA
Cepheid's Combo COVID-19, Flu A/B, RSV Test Earns EUA
Expected to begin shipping to U.S. customers this week.
By PR Newswire09.29.20
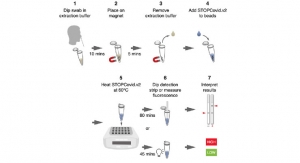
Cepheid (a Danaher business) has received Emergency Use Authorization (EUA) from the U.S. Food & Drug Administration (FDA) for Xpert Xpress SARS-CoV-2/Flu/RSV, a rapid molecular diagnostic test for qualitative detection of the viruses causing COVID-19, Flu A, Flu B, and RSV infections from a single patient sample. The four-in-one test is designed for use on any of Cepheid's over 26,000 GeneXpert Systems placed worldwide, with results delivered in approximately 36 minutes.
"Things may get complicated this respiratory season as clinicians encounter a range of viral infections with symptoms overlapping with COVID-19, including Flu A, Flu B, and respiratory syncytial virus," said Dr. David Persing, MD, Ph.D., Chief Medical and Technology Officer at Cepheid. "The ability to run a single, highly sensitive test that detects all four viruses in a syndromic panel provides actionable results and helps to alleviate pressure on our healthcare system."
Cepheid's previously announced capacity expansion program, supported by parent company Danaher Corporation, was designed in part to address anticipated demand for Xpert Xpress SARS-CoV-2/Flu/RSV. Cepheid expects the first impact of the program in the fourth quarter of 2020 with additional capacity ramping through 2021.
"The dramatic impact of SARS-CoV-2 has been felt by us all, and we understand that a reliable supply of SARS-CoV-2 tests is critical to the communities our healthcare institutions serve—for the coming Flu season and beyond," said Cepheid President Warren Kocmond. "Another goal of the capacity expansion program is to ensure supply continuity of not only our 4-Plex combination test for SARS-CoV-2, Flu A&B and RSV, but the entire portfolio of critical tests Cepheid supplies—including tests for tuberculosis, MRSA, C. difficile, CT/NG, Strep A, and many more."
The new EUA combination test is expected to begin shipping to US customers this week, with expected CE-IVD availability in November.
"Things may get complicated this respiratory season as clinicians encounter a range of viral infections with symptoms overlapping with COVID-19, including Flu A, Flu B, and respiratory syncytial virus," said Dr. David Persing, MD, Ph.D., Chief Medical and Technology Officer at Cepheid. "The ability to run a single, highly sensitive test that detects all four viruses in a syndromic panel provides actionable results and helps to alleviate pressure on our healthcare system."
Cepheid's previously announced capacity expansion program, supported by parent company Danaher Corporation, was designed in part to address anticipated demand for Xpert Xpress SARS-CoV-2/Flu/RSV. Cepheid expects the first impact of the program in the fourth quarter of 2020 with additional capacity ramping through 2021.
"The dramatic impact of SARS-CoV-2 has been felt by us all, and we understand that a reliable supply of SARS-CoV-2 tests is critical to the communities our healthcare institutions serve—for the coming Flu season and beyond," said Cepheid President Warren Kocmond. "Another goal of the capacity expansion program is to ensure supply continuity of not only our 4-Plex combination test for SARS-CoV-2, Flu A&B and RSV, but the entire portfolio of critical tests Cepheid supplies—including tests for tuberculosis, MRSA, C. difficile, CT/NG, Strep A, and many more."
The new EUA combination test is expected to begin shipping to US customers this week, with expected CE-IVD availability in November.
Related Searches: