Business Wire08.29.19
Boyd Corporation, a global developer of sealing, protection and thermal management solutions, announced its facility in Chonburi, Thailand, has achieved certification to the International Organization for Standardization (ISO) Quality Standard 13485:2016 for its Quality Management System (QMS) in place for the medical device industry. Boyd Thailand’s new medical certification expands its ability to serve global medical device and wearable companies with high volume precision converting technologies in best cost geographies.
ISO 13485 is the highest internationally recognized quality standard for organizations involved in the Medical industry. By obtaining this certification, the Thailand facility joins Boyd’s robust list of ISO 13485 medical certified facilities in the United States, Germany, and China. “Attaining this certification demonstrates our global commitment to quality, to meeting customer and international regulatory requirements, as well as helping our customers enhance the safety and reliability of their medical devices,” said Vice-President of Global Medical Sales Fred Knox. “We are very pleased to be poised to supply medical customers with replicable manufacturing technologies in a growing global medical footprint, allowing Boyd to flex and scale with customers as they ramp or penetrate new geographic regions.”
Boyd Thailand’s facility features high-speed, multi-station rotary and flat-bed converting technologies for both mass production and quick-turn prototyping. The Boyd Thailand team is well-equipped to support highly complex converted components with tolerance control and accelerated new product development and design cycles with short lead times. The facility also features clean rooms classified from 1K to 100K and a quality lab suited for measuring and testing, sensitive material handling, and manufacturing of products with stringent cleanliness requirements.
“We are proud to have good people, reliable materials, and exacting processes that continually exceed our Medical customers’ quality and environmental goals on both a strategic and day-to-day work order level,” stated Boyd CEO Mitch Aiello. Boyd’s diverse and complex solutions drive value to global customers by optimizing product performance and efficiency, preventing unintended device failure, minimizing wear and tear, and extending product lifecycles with a design velocity that accelerates development time-to-market, available on three continents.
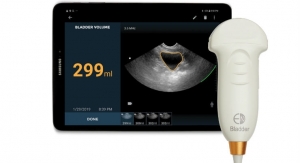
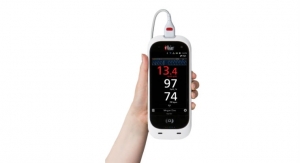
Boyd Corporation’s medical industry support extends from precision converted stick-to-skin medical grade adhesives for wound care and disposables, to lightweight thermal management solutions for medical wearables and portable medical devices, to cooling and sealing MRI machines. With decades of medical expertise and broad design and engineering support, our team can help optimize product design, exceed industry standards and improve product quality. Boyd meets manufacturing needs from ISO 13485 certified facilities on three continents featuring cleanrooms ranging from Class 100 to Class 100K, and established sterilization procedures.
Learn more about Boyd Medical Solutions by watching this video.
Boyd Corporation is a global provider of sealing, protection and thermal management solutions critical to products that keep the world running. The company operates in markets around the world with specific expertise in engineering and design, manufacturing and supply chain management and commits to proactive customer satisfaction across electronics, mobile computing, medical technology, transportation, aerospace and other B2B and consumer-critical industries.
ISO 13485 is the highest internationally recognized quality standard for organizations involved in the Medical industry. By obtaining this certification, the Thailand facility joins Boyd’s robust list of ISO 13485 medical certified facilities in the United States, Germany, and China. “Attaining this certification demonstrates our global commitment to quality, to meeting customer and international regulatory requirements, as well as helping our customers enhance the safety and reliability of their medical devices,” said Vice-President of Global Medical Sales Fred Knox. “We are very pleased to be poised to supply medical customers with replicable manufacturing technologies in a growing global medical footprint, allowing Boyd to flex and scale with customers as they ramp or penetrate new geographic regions.”
Boyd Thailand’s facility features high-speed, multi-station rotary and flat-bed converting technologies for both mass production and quick-turn prototyping. The Boyd Thailand team is well-equipped to support highly complex converted components with tolerance control and accelerated new product development and design cycles with short lead times. The facility also features clean rooms classified from 1K to 100K and a quality lab suited for measuring and testing, sensitive material handling, and manufacturing of products with stringent cleanliness requirements.
“We are proud to have good people, reliable materials, and exacting processes that continually exceed our Medical customers’ quality and environmental goals on both a strategic and day-to-day work order level,” stated Boyd CEO Mitch Aiello. Boyd’s diverse and complex solutions drive value to global customers by optimizing product performance and efficiency, preventing unintended device failure, minimizing wear and tear, and extending product lifecycles with a design velocity that accelerates development time-to-market, available on three continents.
Boyd Corporation’s medical industry support extends from precision converted stick-to-skin medical grade adhesives for wound care and disposables, to lightweight thermal management solutions for medical wearables and portable medical devices, to cooling and sealing MRI machines. With decades of medical expertise and broad design and engineering support, our team can help optimize product design, exceed industry standards and improve product quality. Boyd meets manufacturing needs from ISO 13485 certified facilities on three continents featuring cleanrooms ranging from Class 100 to Class 100K, and established sterilization procedures.
Learn more about Boyd Medical Solutions by watching this video.
Boyd Corporation is a global provider of sealing, protection and thermal management solutions critical to products that keep the world running. The company operates in markets around the world with specific expertise in engineering and design, manufacturing and supply chain management and commits to proactive customer satisfaction across electronics, mobile computing, medical technology, transportation, aerospace and other B2B and consumer-critical industries.