Bob Bordignon, Vice President of Global Sales, MGS07.30.19
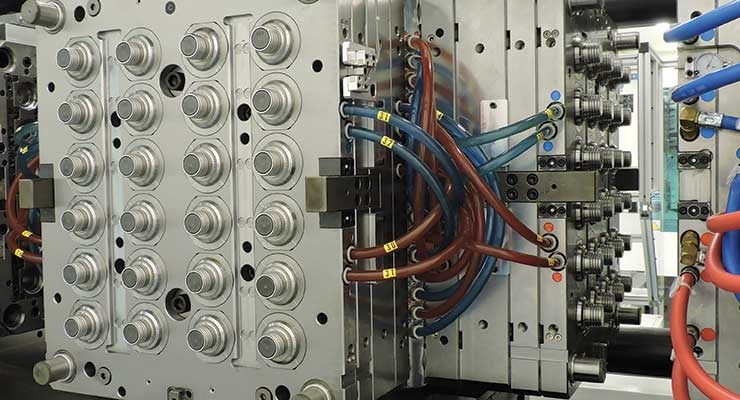
Today’s medical device OEMs are faced with two major choices when it comes to molding custom plastic components: partner with a custom injection molder or build out the capacity in-house, working with the supply chain as needed to ensure the use of the right tools, design processes, and integration of secondary operations.
Some OEMs choose to mold parts and products in-house to maintain control over processes, protect intellectual property, and integrate into the larger work stream. Many often struggle, however, to maintain the proper level of resource investment necessary given the capital-intense nature of the injection molding business.
Frequently, the original decision to mold in-house to save costs, streamline the supply chain, and protect intellectual property causes OEMs to be left scrambling when things go wrong. Whether the program gets derailed by poor quality tooling, the wrong downstream equipment, or a lack of ongoing investment and resources, it’s important to ask, “Is it time to outsource our molding business?”
Members of management at OEMs struggling with the answer to this question should try to break down the current situation:
Managing the Ongoing Investment
When making the initial decision of how to manufacture plastic molded parts, there are plenty of positives to bringing the work in-house. You alone have control over the investment, part quality, timeline, and potential integration into the larger manufacturing operation. For some OEMs, molding programs continue to be a significant part of their ongoing competencies and business performance. However, with growing complexity, pressure to get to market faster, and a need for positive financial performance, managing in-house injection molding operations may prove detrimental to the long-term success of the company’s programs.
It can be easy for many companies to overlook how resource investments could be better spent helping gain a bigger market advantage. To understand this, companies must review the “make vs. buy” decision holistically to understand the best strategy for them.
Complexity in Making the ‘Make’ Decision
In understanding the “make vs. buy” decision, OEMs often decide to maintain control of their molding operations as they feel it represents a lower-cost, more efficient way to get plastic parts manufactured. There are three key questions, however, that managers at these businesses should ask themselves prior to making the decision:
Maintaining Control Over Intellectual Property
In the highly competitive medical product industry, intellectual property (IP) can be critical to maintaining a firm hold on market share. As such, initial decisions to bring molding operations in-house are often based on a strategy that further ensures protection of IP, which can be deeply embedded in the design or manufacturing process of the plastic parts. In those cases, further insurance of proper protection can be gained by molding in-house.
OEMs, however, must be sure to understand ways in which IP can be protected throughout the outsourcing partnership. Understand the IP assets, the risks involved (e.g., loss of market share), and the best way to protect those assets (e.g., non-disclosure agreements, strong contracts, thoroughly vetting the outsource partner, etc.). Weighing these concerns with the overall benefit of outsourcing will help managers factor in the value of partnering with a custom molder.
Investing in Human Resources
A robust injection molding operation requires significant human resource investments and support. As top talent in the industry becomes harder to find and keep, OEMs struggle to attract candidates and justify the increasing costs of human capital. The conflict comes into play when deciding whether to hire plastics processing experts versus hiring product designers, R&D engineers, or chemists who more closely tie to the OEM’s core business.
Further, in-house molding programs can also inhibit the existing team’s ability to drive new, innovative programs. As most brand owners continue to focus on new product introductions that leverage their brand and distribution channels, the conversion of pellets into parts becomes an added distraction and strain on the organization. The question then becomes, “Should I be a better molder, or a product innovator?” The asking and/or answering of that question often triggers the exploration into the outsourcing of OEM molding operations to better enable the team to fill that role.
Uncovering the True Costs of Molding In-House
Initial decisions to bring operations in-house are often based upon a strategy that seeks a low-cost, efficient way to get quality plastic parts manufactured. Once these lines are up and running and initial startup costs (e.g., new equipment, tooling, materials, and staff) have been invested, however, OEMs may discover they’re experiencing an unexpected drain on their internal resources.
Throughout the lifecycle of the molding operations, additional costs arise that can include ongoing capital investment, maintenance costs for general upkeep, added resources needed when processes malfunction, additional time working with third parties to troubleshoot, and more. For OEMs with complex, critical parts, these areas must be the focus of continuous reinvestment to sustain high-quality performance.
Throughout this process, financials are analyzed and senior leaders weigh in, placing increased pressure on making the right decision. For larger OEM organizations, costs can be allocated to many departments and are difficult to accurately assign to the total cost of molding plastics parts. For example, tooling engineering to support a molding site may be absorbed as a corporate charge instead of being accounted for as a cost to mold. This can create discrepancies when assessing whether or not to outsource molding, and leaves the decision maker with an incomplete picture of current costs versus savings. Once total actual costs are captured, a savings in outsourcing is normally identified.
Understanding the Capital Investment
Once the commitment needed to “make” from a general cost and strategy perspective is understood, it’s important to recognize the specifics of the expenditures required, since the capital investment necessary to set up and maintain a competitive molding operation is significant. From ensuring the current facilities are well-equipped to managing the new production line to understanding if additional investment in machinery or facilities is needed, the capital investment related to in-house molding should not be considered lightly.
The processing and handling of plastic parts typically requires a large footprint, decreasing an OEM’s ability to expand successful, innovative product lines and add new ones. This increasingly impacts companies in the face of rapid growth and consolidation in the industry, which is causing medical device and product manufacturers to consolidate product lines, businesses, and processes at an unprecedented pace. In outsourcing molded parts, an OEM is freeing up capacity of both the team and the facility to better manage product lines within a capital-intensive space. Additionally, in-house molding also consumes considerable energy. Regions with high energy costs and venerable supply add an additional element of risk to the cost model.
Breaking Down the Investment
As an example, MGS worked with a leading pharmaceutical company which did the cost modeling to retool an outdated and non-compliant facility that annually manufactured 2 billion small components for a drug delivery device. The initial cost to rebuild was estimated to be $30 million for the OEM. What the analysis didn’t take into consideration, however, was future annual investment, which could have reached seven figures. Of course, this cost also increases as the original investment ages.
This reality initiated a “make vs. buy” evaluation, while illustrating that outsourcing would be a beneficial option. What would be a significant internal investment for a medical device OEM, in comparison, is simply considered “table stakes” to a custom molder who partners with medtech firms.
Designing a Better Way
Within the highly regulated landscape of medical product manufacturing, the molding environment, quality systems, and risk mitigation necessary in the manufacturing processes can also be a concern. Not too long ago, these concepts were foreign to the custom plastic molding industry. This fact forced healthcare OEMs to set up and run their plastic parts in-house as an extension of their manufacturing capabilities.
The acceptance and expansion of groups like the International Organization for Standardization (ISO) coupled with plastic industry pioneers investing in the necessary tools to safely mold medical parts has led to an abundance of capable outsourcing options. We have gone from an era where there were no options to today’s environment rich with capable suppliers.
Because of this, the custom molder and OEM partnership is more important than ever before. Today, the opportunity exists to create an integrated supply chain partnership that enables an OEM to protect its intellectual property, manage costs, ensure ongoing operational excellence, take care of the ability to leverage investments of people, and be a leading firm within the community.
Additional benefits outside of manufacturing costs savings can be realized by working with an outsource partner. Time to market can be reduced by leveraging a vertically integrated prototype/production mold builder, molder, and secondary operations provider who understands the pace necessary to launch new products on time as well as process efficiency techniques like design for manufacturability.
The right custom molder services multiple industries and processes, as that cross-pollination of experience can be leveraged to provide new insights into product design, the manufacturing process, and sustainability options. The value a custom molder can provide is worth a second look as options are analyzed.
Evaluating the ‘Buy’ Decision
Once the decision to outsource the molding business has been made, the next major decision is who to outsource it to. Choosing the right partner is a complex process in decision making. Further, since the medical molding landscape is crowded with many injection molders—and not all are created equal—the decision is anything but simple.
A contract molder with internal horsepower in tooling, molding equipment, and automation can more easily overcome major challenges—like ongoing capital investments, troubleshooting, and designing the right process—and remedy situations swiftly. Whether launching a new part or seeking to improve existing product lines, consider the information needed to make the right decision.
Simplifying Complexity with the Right Custom Molder
Once a thorough evaluation of the barriers to molding a part in-house has been performed—ongoing resource investments, intellectual property, personnel concerns—it’s important to do the same evaluation of outsourcing molded parts. OEMs must consider the following factors when evaluating potential partners:
Experience: Finding a partner with an extensive body of work in the field of integrating large transfer projects into their facilities or taking over yours is critical. Many custom molders will also excel at supporting new product introductions. This experience helps molders specify the right process for a company’s specific needs.
Extensive Capabilities: Choosing a custom molder that is vertically integrated is also important for success. Finding a partner who builds tooling, automation, and also molds parts in an ISO-compliant environment—and can help manage FDA approvals—will help drive value for a project.
Streamlined Supply Chain: Greater cost savings can be realized simply from streamlining the supply chain through a successful relationship with the right molder. The right partner will be able to act as a single source of supply, helping to design a part for manufacturability, build the right mold, select the right materials, mold the part, and provide any secondary operations required.
Accountability: Within a streamlined supply chain, the accountability of an outsource partner will increase. If an issue with the molded parts exists, it’s fairly obvious whom to turn to for assistance to minimize risk and increase accountability.
Not just any custom molder will bring together these four elements to support a company’s need for high-quality parts. It’s important to thoroughly vet each custom molder to ensure the right “make vs. buy” decision.
Case in Point
The need to make the outsourcing decision can be required for many reasons. For example, recently, a large medical device and pharmaceutical company with extensive internal molding capabilities spun off a division as its own company under a new name. During the split, the new entity did not retain any facilities with injection molding capabilities, presenting them with a significant decision—to set up shop or outsource.
The contract was written so that the original OEM was responsible for continuing to supply molded components for a maximum period of two years. The criteria included an ISO Class 8 cleanroom environment, 16 molding machines, 24/7 operations, a fully staffed and equipped mold maintenance department with a fleet of 46 molds ranging from 10 to 25 years old, and some secondary operations.
Since the parts were technically challenging and the molds were complex and aged, needing maintenance and repair, the company chose to outsource the molding business, selecting MGS based upon tooling horsepower and a proven track record in healthcare transfer molding. The transfer, mold repair, and validation work was 100 percent complete in six months, allowing the OEM to fulfill its commitment without the complexity associated with keeping the program in-house.
Conclusion
The “make vs. buy” decision is not one that should be considered lightly. There are significant expenses involved and lapses in quality cannot occur. As such, medical device OEMs should take time to perform the required due diligence to ensure they are making the correct choice for their specific business needs.
Bob Bordignon is vice president of global sales for MGS. He has more than 30 years of experience in custom plastics manufacturing.
Some OEMs choose to mold parts and products in-house to maintain control over processes, protect intellectual property, and integrate into the larger work stream. Many often struggle, however, to maintain the proper level of resource investment necessary given the capital-intense nature of the injection molding business.
Frequently, the original decision to mold in-house to save costs, streamline the supply chain, and protect intellectual property causes OEMs to be left scrambling when things go wrong. Whether the program gets derailed by poor quality tooling, the wrong downstream equipment, or a lack of ongoing investment and resources, it’s important to ask, “Is it time to outsource our molding business?”
Members of management at OEMs struggling with the answer to this question should try to break down the current situation:
- Do you find yourself struggling to maintain an ongoing investment in the operations—especially in cutting-edge molding and tooling technologies that can enhance your product offering?
- Is a lack of reinvestment in your molding operations standing in the way of consistent quality and delivery?
- Are you missing opportunities to allow your employees to innovate and drive greater success because their time is spent managing or troubleshooting molding?
- Are you doubting your ability to hit your profitability targets?
Managing the Ongoing Investment
When making the initial decision of how to manufacture plastic molded parts, there are plenty of positives to bringing the work in-house. You alone have control over the investment, part quality, timeline, and potential integration into the larger manufacturing operation. For some OEMs, molding programs continue to be a significant part of their ongoing competencies and business performance. However, with growing complexity, pressure to get to market faster, and a need for positive financial performance, managing in-house injection molding operations may prove detrimental to the long-term success of the company’s programs.
It can be easy for many companies to overlook how resource investments could be better spent helping gain a bigger market advantage. To understand this, companies must review the “make vs. buy” decision holistically to understand the best strategy for them.
Complexity in Making the ‘Make’ Decision
In understanding the “make vs. buy” decision, OEMs often decide to maintain control of their molding operations as they feel it represents a lower-cost, more efficient way to get plastic parts manufactured. There are three key questions, however, that managers at these businesses should ask themselves prior to making the decision:
- Am I concerned that I won’t maintain control over my intellectual property?
- Are we leveraging our team in the best way possible to set our employees and our company up for success?
- Have I considered all of the costs that will be needed to maintain the high level of performance needed for this product?
Maintaining Control Over Intellectual Property
In the highly competitive medical product industry, intellectual property (IP) can be critical to maintaining a firm hold on market share. As such, initial decisions to bring molding operations in-house are often based on a strategy that further ensures protection of IP, which can be deeply embedded in the design or manufacturing process of the plastic parts. In those cases, further insurance of proper protection can be gained by molding in-house.
OEMs, however, must be sure to understand ways in which IP can be protected throughout the outsourcing partnership. Understand the IP assets, the risks involved (e.g., loss of market share), and the best way to protect those assets (e.g., non-disclosure agreements, strong contracts, thoroughly vetting the outsource partner, etc.). Weighing these concerns with the overall benefit of outsourcing will help managers factor in the value of partnering with a custom molder.
Investing in Human Resources
A robust injection molding operation requires significant human resource investments and support. As top talent in the industry becomes harder to find and keep, OEMs struggle to attract candidates and justify the increasing costs of human capital. The conflict comes into play when deciding whether to hire plastics processing experts versus hiring product designers, R&D engineers, or chemists who more closely tie to the OEM’s core business.
Further, in-house molding programs can also inhibit the existing team’s ability to drive new, innovative programs. As most brand owners continue to focus on new product introductions that leverage their brand and distribution channels, the conversion of pellets into parts becomes an added distraction and strain on the organization. The question then becomes, “Should I be a better molder, or a product innovator?” The asking and/or answering of that question often triggers the exploration into the outsourcing of OEM molding operations to better enable the team to fill that role.
Uncovering the True Costs of Molding In-House
Initial decisions to bring operations in-house are often based upon a strategy that seeks a low-cost, efficient way to get quality plastic parts manufactured. Once these lines are up and running and initial startup costs (e.g., new equipment, tooling, materials, and staff) have been invested, however, OEMs may discover they’re experiencing an unexpected drain on their internal resources.
Throughout the lifecycle of the molding operations, additional costs arise that can include ongoing capital investment, maintenance costs for general upkeep, added resources needed when processes malfunction, additional time working with third parties to troubleshoot, and more. For OEMs with complex, critical parts, these areas must be the focus of continuous reinvestment to sustain high-quality performance.
Throughout this process, financials are analyzed and senior leaders weigh in, placing increased pressure on making the right decision. For larger OEM organizations, costs can be allocated to many departments and are difficult to accurately assign to the total cost of molding plastics parts. For example, tooling engineering to support a molding site may be absorbed as a corporate charge instead of being accounted for as a cost to mold. This can create discrepancies when assessing whether or not to outsource molding, and leaves the decision maker with an incomplete picture of current costs versus savings. Once total actual costs are captured, a savings in outsourcing is normally identified.
Understanding the Capital Investment
Once the commitment needed to “make” from a general cost and strategy perspective is understood, it’s important to recognize the specifics of the expenditures required, since the capital investment necessary to set up and maintain a competitive molding operation is significant. From ensuring the current facilities are well-equipped to managing the new production line to understanding if additional investment in machinery or facilities is needed, the capital investment related to in-house molding should not be considered lightly.
The processing and handling of plastic parts typically requires a large footprint, decreasing an OEM’s ability to expand successful, innovative product lines and add new ones. This increasingly impacts companies in the face of rapid growth and consolidation in the industry, which is causing medical device and product manufacturers to consolidate product lines, businesses, and processes at an unprecedented pace. In outsourcing molded parts, an OEM is freeing up capacity of both the team and the facility to better manage product lines within a capital-intensive space. Additionally, in-house molding also consumes considerable energy. Regions with high energy costs and venerable supply add an additional element of risk to the cost model.
Breaking Down the Investment
As an example, MGS worked with a leading pharmaceutical company which did the cost modeling to retool an outdated and non-compliant facility that annually manufactured 2 billion small components for a drug delivery device. The initial cost to rebuild was estimated to be $30 million for the OEM. What the analysis didn’t take into consideration, however, was future annual investment, which could have reached seven figures. Of course, this cost also increases as the original investment ages.
This reality initiated a “make vs. buy” evaluation, while illustrating that outsourcing would be a beneficial option. What would be a significant internal investment for a medical device OEM, in comparison, is simply considered “table stakes” to a custom molder who partners with medtech firms.
Designing a Better Way
Within the highly regulated landscape of medical product manufacturing, the molding environment, quality systems, and risk mitigation necessary in the manufacturing processes can also be a concern. Not too long ago, these concepts were foreign to the custom plastic molding industry. This fact forced healthcare OEMs to set up and run their plastic parts in-house as an extension of their manufacturing capabilities.
The acceptance and expansion of groups like the International Organization for Standardization (ISO) coupled with plastic industry pioneers investing in the necessary tools to safely mold medical parts has led to an abundance of capable outsourcing options. We have gone from an era where there were no options to today’s environment rich with capable suppliers.
Because of this, the custom molder and OEM partnership is more important than ever before. Today, the opportunity exists to create an integrated supply chain partnership that enables an OEM to protect its intellectual property, manage costs, ensure ongoing operational excellence, take care of the ability to leverage investments of people, and be a leading firm within the community.
Additional benefits outside of manufacturing costs savings can be realized by working with an outsource partner. Time to market can be reduced by leveraging a vertically integrated prototype/production mold builder, molder, and secondary operations provider who understands the pace necessary to launch new products on time as well as process efficiency techniques like design for manufacturability.
The right custom molder services multiple industries and processes, as that cross-pollination of experience can be leveraged to provide new insights into product design, the manufacturing process, and sustainability options. The value a custom molder can provide is worth a second look as options are analyzed.
Evaluating the ‘Buy’ Decision
Once the decision to outsource the molding business has been made, the next major decision is who to outsource it to. Choosing the right partner is a complex process in decision making. Further, since the medical molding landscape is crowded with many injection molders—and not all are created equal—the decision is anything but simple.
A contract molder with internal horsepower in tooling, molding equipment, and automation can more easily overcome major challenges—like ongoing capital investments, troubleshooting, and designing the right process—and remedy situations swiftly. Whether launching a new part or seeking to improve existing product lines, consider the information needed to make the right decision.
Simplifying Complexity with the Right Custom Molder
Once a thorough evaluation of the barriers to molding a part in-house has been performed—ongoing resource investments, intellectual property, personnel concerns—it’s important to do the same evaluation of outsourcing molded parts. OEMs must consider the following factors when evaluating potential partners:
Experience: Finding a partner with an extensive body of work in the field of integrating large transfer projects into their facilities or taking over yours is critical. Many custom molders will also excel at supporting new product introductions. This experience helps molders specify the right process for a company’s specific needs.
Extensive Capabilities: Choosing a custom molder that is vertically integrated is also important for success. Finding a partner who builds tooling, automation, and also molds parts in an ISO-compliant environment—and can help manage FDA approvals—will help drive value for a project.
Streamlined Supply Chain: Greater cost savings can be realized simply from streamlining the supply chain through a successful relationship with the right molder. The right partner will be able to act as a single source of supply, helping to design a part for manufacturability, build the right mold, select the right materials, mold the part, and provide any secondary operations required.
Accountability: Within a streamlined supply chain, the accountability of an outsource partner will increase. If an issue with the molded parts exists, it’s fairly obvious whom to turn to for assistance to minimize risk and increase accountability.
Not just any custom molder will bring together these four elements to support a company’s need for high-quality parts. It’s important to thoroughly vet each custom molder to ensure the right “make vs. buy” decision.
Case in Point
The need to make the outsourcing decision can be required for many reasons. For example, recently, a large medical device and pharmaceutical company with extensive internal molding capabilities spun off a division as its own company under a new name. During the split, the new entity did not retain any facilities with injection molding capabilities, presenting them with a significant decision—to set up shop or outsource.
The contract was written so that the original OEM was responsible for continuing to supply molded components for a maximum period of two years. The criteria included an ISO Class 8 cleanroom environment, 16 molding machines, 24/7 operations, a fully staffed and equipped mold maintenance department with a fleet of 46 molds ranging from 10 to 25 years old, and some secondary operations.
Since the parts were technically challenging and the molds were complex and aged, needing maintenance and repair, the company chose to outsource the molding business, selecting MGS based upon tooling horsepower and a proven track record in healthcare transfer molding. The transfer, mold repair, and validation work was 100 percent complete in six months, allowing the OEM to fulfill its commitment without the complexity associated with keeping the program in-house.
Conclusion
The “make vs. buy” decision is not one that should be considered lightly. There are significant expenses involved and lapses in quality cannot occur. As such, medical device OEMs should take time to perform the required due diligence to ensure they are making the correct choice for their specific business needs.
Bob Bordignon is vice president of global sales for MGS. He has more than 30 years of experience in custom plastics manufacturing.