07.15.19
BellaSeno GmbH, a company developing absorbable implants using additive manufacturing technology, announced it obtained full ISO 13485 certification for its design and additive manufacturing processes, ranging from the concept and prototype to the production of novel, non-silicone-based absorbable implants. In addition, the company raised a further €1 million from its existing investors, bringing the total amount raised by BellaSeno to €4.2 million. The proceeds will be used to fund a clinical trial of Senella, its first product, a biocompatible and fully absorbable breast scaffold made from a polymer that is FDA-approved and CE-marked for a wide variety of clinical applications. The trial is scheduled to start in Q4/2019.
“We are now one of very few companies worldwide with an ISO 13485 certification covering the entire process of design and additive manufacturing of absorbable implants,” said Dr. Simon Champ, CEO of BellaSeno. “This is not only a prerequisite for the clinical trial of Senella, our first product, but also for closing contract manufacturing deals with companies seeking to develop and market their own absorbable implants for a wide range of medical applications. We are offering the entire process under ISO 13485 certification to other medtech companies worldwide—from concept and inhouse design to manufacturing of prototypes, clinical trials, and series production.”
He added that BellaSeno’s platform technology and patent-pending porous architectures can be used to design and manufacture a broad spectrum of soft and stiff devices for bone and soft tissue replacements.
Absorbable implants guide the growth of natural tissue derived from the patient's own body and are slowly, but fully absorbed over a period of several days, months or years, dependent on the composition of the polymer employed. In contrast to many other implants, the result is a natural tissue with no foreign body remnants. BellaSeno’s implants are produced via additive manufacturing (AM), more commonly known as 3D-printing.
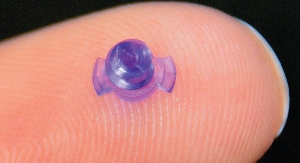
Senella, is a patented, porous, absorbable scaffold which will provide a step change in the quality of life of breast reconstruction and augmentation patients around the world. The company believes it has the potential to disrupt the current breast surgery markets, which at present are dominated by silicone implants.
“We are now one of very few companies worldwide with an ISO 13485 certification covering the entire process of design and additive manufacturing of absorbable implants,” said Dr. Simon Champ, CEO of BellaSeno. “This is not only a prerequisite for the clinical trial of Senella, our first product, but also for closing contract manufacturing deals with companies seeking to develop and market their own absorbable implants for a wide range of medical applications. We are offering the entire process under ISO 13485 certification to other medtech companies worldwide—from concept and inhouse design to manufacturing of prototypes, clinical trials, and series production.”
He added that BellaSeno’s platform technology and patent-pending porous architectures can be used to design and manufacture a broad spectrum of soft and stiff devices for bone and soft tissue replacements.
Absorbable implants guide the growth of natural tissue derived from the patient's own body and are slowly, but fully absorbed over a period of several days, months or years, dependent on the composition of the polymer employed. In contrast to many other implants, the result is a natural tissue with no foreign body remnants. BellaSeno’s implants are produced via additive manufacturing (AM), more commonly known as 3D-printing.
Senella, is a patented, porous, absorbable scaffold which will provide a step change in the quality of life of breast reconstruction and augmentation patients around the world. The company believes it has the potential to disrupt the current breast surgery markets, which at present are dominated by silicone implants.