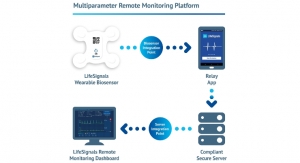
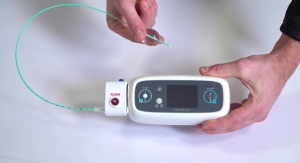
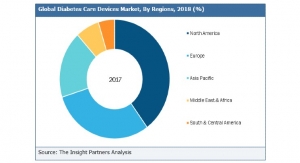
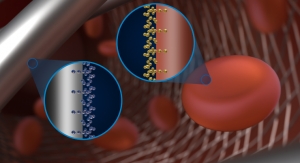
Business Wire05.18.18
MED-EL USA announced the U.S. Food and Drug Administration (FDA) approval of the RONDO 2 cochlear implant (CI) audio processor featuring revolutionary wireless charging. Users simply place the processor on the charge pad and it will charge automatically without wires or cables, negating the need for disposable batteries. The rechargeable battery provides up to 18 hours of battery life, enabling all-day hearing. The battery is fully recharged after just four hours.
Along with its innovative wireless charging, RONDO 2 has a sleek design with just one simple on/off button and automatic volume control. It easily connects to cell phones and televisions using intelligent wireless accessories, and connects seamlessly with Bluetooth neckloops and hearing induction loops, even across a busy room. The compact design means it can be hidden discreetly under hair.
“RONDO 2 is the easiest to use CI audio processor ever made,” said Raymond Gamble, CEO & President, MED-EL North America. “And because MED-EL engineers every internal cochlear implant to be future-ready, all of our recipients will be able to enjoy the latest industry-leading audio processing technology.”
The new processor also includes features that allow users to easily tailor their RONDO 2 to their personal style and lifestyle. Recipients can customize their RONDO 2 to blend in with five neutral colors and hair patterned design covers, or make a statement with colorful and art-inspired designs and wild animal prints. RONDO 2’s IP54 rating means it is protected from everyday splashes and moisture. For full submersion, WaterWear reusable waterproof covers are easy to attach and can be used in any type of water, including salty seawater and chlorinated swimming pools.
Product availability is estimated in Fall 2018. Recipients who choose a MED-EL Cochlear Implant between now and availability will receive a voucher for a FREE RONDO 2 with the PlusRONDO promotion. All new MED-EL CI recipients have the option to experience two ways to hear: the freedom of nothing behind the ear with an all-in-one, cable-free design, with the RONDO 2, and the exceptional performance of the SONNET Audio Processor—all backed by MED-EL’s industry-leading five-year warranty. New recipients also benefit from the outstanding reliability and exceptional hearing performance of the SYNCHRONY Cochlear Implant.
Along with its innovative wireless charging, RONDO 2 has a sleek design with just one simple on/off button and automatic volume control. It easily connects to cell phones and televisions using intelligent wireless accessories, and connects seamlessly with Bluetooth neckloops and hearing induction loops, even across a busy room. The compact design means it can be hidden discreetly under hair.
“RONDO 2 is the easiest to use CI audio processor ever made,” said Raymond Gamble, CEO & President, MED-EL North America. “And because MED-EL engineers every internal cochlear implant to be future-ready, all of our recipients will be able to enjoy the latest industry-leading audio processing technology.”
The new processor also includes features that allow users to easily tailor their RONDO 2 to their personal style and lifestyle. Recipients can customize their RONDO 2 to blend in with five neutral colors and hair patterned design covers, or make a statement with colorful and art-inspired designs and wild animal prints. RONDO 2’s IP54 rating means it is protected from everyday splashes and moisture. For full submersion, WaterWear reusable waterproof covers are easy to attach and can be used in any type of water, including salty seawater and chlorinated swimming pools.
Product availability is estimated in Fall 2018. Recipients who choose a MED-EL Cochlear Implant between now and availability will receive a voucher for a FREE RONDO 2 with the PlusRONDO promotion. All new MED-EL CI recipients have the option to experience two ways to hear: the freedom of nothing behind the ear with an all-in-one, cable-free design, with the RONDO 2, and the exceptional performance of the SONNET Audio Processor—all backed by MED-EL’s industry-leading five-year warranty. New recipients also benefit from the outstanding reliability and exceptional hearing performance of the SYNCHRONY Cochlear Implant.