Keystone Heart Ltd. 03.14.17
Keystone Heart Ltd., a developer of cerebral embolic protection devices for patients undergoing cardiac procedures, and Venus Medtech (Hangzhou) Inc., a Chinese transcatheter heart valve company, have forged a partnership agreement to provide Venus Medtech’s TAVR system in combination with Keystone Heart’s TriGuard Cerebral Embolic Protection Device. This agreement, covering China and key Asian markets, will allow physicians and patients in this region to benefit from TAVR while simultaneously receiving complete three vessel embolic protection for the brain. The combination is unavailable in any other market or from any other company.
“It is of utmost importance to us that our devices improve the quality life of the patients whom receive them,” said Eric Zi, president and CEO of Venus Medtech. “Our transcatheter heart valve systems offer patients life-saving support— and collaborating with Keystone Heart allows us the opportunity to reduce the risk of brain injury during the procedure. No other company offers this combination.”
In February 2017, the Neurologic Academic Research Consortium (NeuroARC) published formal guidelines that establish and provide standardized consensus definitions for neurologic endpoints in cardiovascular clinical trials. Released in the Journal of American College of Cardiology and the European Heart Journal, these guidelines highlight a growing body of evidence pointing to the existence of “covert” brain injury to patients undergoing transcatheter aortic valve replacement (TAVR) and other cardiovascular procedures.
Highlighting this issue, over the last five years, a total of 13 studies conducted in the United States and Europe have shown that a median of 80 percent of patients have new brain lesions following TAVR. In recent research in U.S. institutions, the NeuroTAVR study demonstrated that 94 percent of patients had new lesions in the brain following the TAVR procedure, 22.6 percent of patients had new neurologic impairment post TAVR, and 41 percent of patients had declining neurocognition at 30 days when compared to pre-TAVR scores in the United States.
“Providing brain protection for every TAVR patient will differentiate Venus Medtech and position them as a leader in structural heart therapies—underscoring its dedication to excellence and focus on patient safety,” said Chris Richardson, president and CEO of Keystone Heart. “This partnership provides us the opportunity to make an impact in the fast-growing structural heart space in an important geographic market.”
China is an emerging market for TAVR procedures. Venus Medtech expects its TAVR device will be the first device approved by the China Food and Drug Administration for marketing in China. Experts predict that there will be more than 10,000 procedures conducted in the first three years following approval and the market is expected to grow 30 percent to 50 percent per year in the following years.
TAVR, which is also referred to as transcatheter aortic valve implantation (TAVI), is a minimally invasive surgical procedure conducted to repair a damaged aortic valve. A damaged aortic valve obstructs blood flow from the heart into the aorta and onward to the rest of the body, and if left untreated, may lead to death. There will be approximately 125,000 TAVR procedures conducted this year worldwide.
Venus Medtech (HangZhou) Inc. is a heart valve developer in China. Its transcatheter aortic valves have completed clinical trials and follow-up studies, and are pending China Food and Drug Administration clearance. Its transcatheter pulmonic valves are completing clinical trials. Both of the valves are expected to be the first transcatheter valves approved in China. They are also developing the next-generation heart valves designed for increased safety and long term durability.
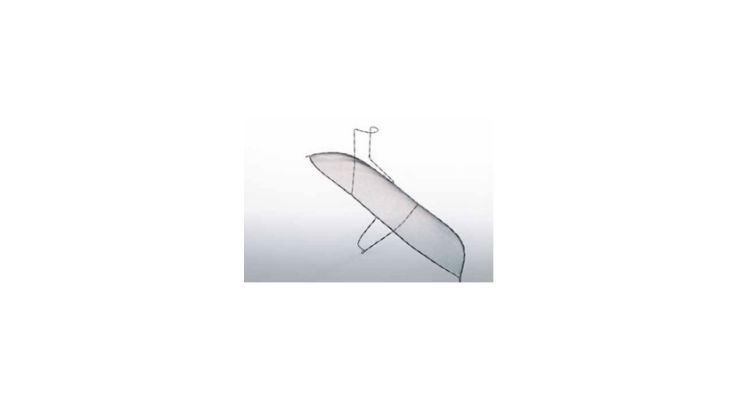
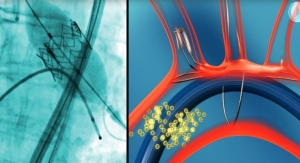
Keystone Heart's TriGuard is the only cerebral protection device designed to provide full coverage to all brain territories to minimize the risk of cerebral damage during TAVR and other cardiovascular procedures. The CE marked TriGuard device is shaped to accommodate anatomic variations of the aortic arch. Formed to withstand potential interface with the TAVR delivery system and other procedure related accessories, it uses a Nitinol frame and mesh—flexible and atraumatic, yet robust and sturdy.
The TriGuard Cerebral Embolic Protection Device is placed through one of two femoral artery access ports typically used in TAVR, thereby eliminating the need for a third puncture site. It deploys rapidly, and self-positions through a small 9F catheter. TriGuard provides stable, atraumatic protection, with simple retrieval.
The TriGuard Cerebral Embolic Protection Device has been granted CE Mark approval in the European Union and is commercially available in Europe. In the United States, the device is currently available for investigational use only.
Keystone Heart Ltd. develops and manufactures cerebral embolic protection devices to reduce the risk of stroke, neurocognitive decline and dementia caused by brain emboli associated with cardiovascular procedures. The company is focused on protecting the brain from emboli to reduce the risk of brain infarcts during TAVR, atrial fibrillation ablation and other cardiovascular procedures. The TriGuard product pipeline is designed to help interventional cardiologists, electrophysiologists and cardiac surgeons to preserve brain reserve while performing these procedures.
The company is headquartered in Israel and has U.S. operations in Tampa, Fla.
“It is of utmost importance to us that our devices improve the quality life of the patients whom receive them,” said Eric Zi, president and CEO of Venus Medtech. “Our transcatheter heart valve systems offer patients life-saving support— and collaborating with Keystone Heart allows us the opportunity to reduce the risk of brain injury during the procedure. No other company offers this combination.”
In February 2017, the Neurologic Academic Research Consortium (NeuroARC) published formal guidelines that establish and provide standardized consensus definitions for neurologic endpoints in cardiovascular clinical trials. Released in the Journal of American College of Cardiology and the European Heart Journal, these guidelines highlight a growing body of evidence pointing to the existence of “covert” brain injury to patients undergoing transcatheter aortic valve replacement (TAVR) and other cardiovascular procedures.
Highlighting this issue, over the last five years, a total of 13 studies conducted in the United States and Europe have shown that a median of 80 percent of patients have new brain lesions following TAVR. In recent research in U.S. institutions, the NeuroTAVR study demonstrated that 94 percent of patients had new lesions in the brain following the TAVR procedure, 22.6 percent of patients had new neurologic impairment post TAVR, and 41 percent of patients had declining neurocognition at 30 days when compared to pre-TAVR scores in the United States.
“Providing brain protection for every TAVR patient will differentiate Venus Medtech and position them as a leader in structural heart therapies—underscoring its dedication to excellence and focus on patient safety,” said Chris Richardson, president and CEO of Keystone Heart. “This partnership provides us the opportunity to make an impact in the fast-growing structural heart space in an important geographic market.”
China is an emerging market for TAVR procedures. Venus Medtech expects its TAVR device will be the first device approved by the China Food and Drug Administration for marketing in China. Experts predict that there will be more than 10,000 procedures conducted in the first three years following approval and the market is expected to grow 30 percent to 50 percent per year in the following years.
TAVR, which is also referred to as transcatheter aortic valve implantation (TAVI), is a minimally invasive surgical procedure conducted to repair a damaged aortic valve. A damaged aortic valve obstructs blood flow from the heart into the aorta and onward to the rest of the body, and if left untreated, may lead to death. There will be approximately 125,000 TAVR procedures conducted this year worldwide.
Venus Medtech (HangZhou) Inc. is a heart valve developer in China. Its transcatheter aortic valves have completed clinical trials and follow-up studies, and are pending China Food and Drug Administration clearance. Its transcatheter pulmonic valves are completing clinical trials. Both of the valves are expected to be the first transcatheter valves approved in China. They are also developing the next-generation heart valves designed for increased safety and long term durability.
Keystone Heart's TriGuard is the only cerebral protection device designed to provide full coverage to all brain territories to minimize the risk of cerebral damage during TAVR and other cardiovascular procedures. The CE marked TriGuard device is shaped to accommodate anatomic variations of the aortic arch. Formed to withstand potential interface with the TAVR delivery system and other procedure related accessories, it uses a Nitinol frame and mesh—flexible and atraumatic, yet robust and sturdy.
The TriGuard Cerebral Embolic Protection Device is placed through one of two femoral artery access ports typically used in TAVR, thereby eliminating the need for a third puncture site. It deploys rapidly, and self-positions through a small 9F catheter. TriGuard provides stable, atraumatic protection, with simple retrieval.
The TriGuard Cerebral Embolic Protection Device has been granted CE Mark approval in the European Union and is commercially available in Europe. In the United States, the device is currently available for investigational use only.
Keystone Heart Ltd. develops and manufactures cerebral embolic protection devices to reduce the risk of stroke, neurocognitive decline and dementia caused by brain emboli associated with cardiovascular procedures. The company is focused on protecting the brain from emboli to reduce the risk of brain infarcts during TAVR, atrial fibrillation ablation and other cardiovascular procedures. The TriGuard product pipeline is designed to help interventional cardiologists, electrophysiologists and cardiac surgeons to preserve brain reserve while performing these procedures.
The company is headquartered in Israel and has U.S. operations in Tampa, Fla.