Business Wire04.03.17
NICO Corporation, a developer of interventional technologies used in a new way of doing brain surgery, announced the CE Mark for BrainPath—a patented technology that provides non-disruptive access to the brain by uniquely using both a parafascicular and trans-sulcal surgical approach. Gaining the CE Mark recognizes BrainPath for use in more than 30 countries in the European Union. The technology has growing demand in the worldwide market where 5.2 million brain tumor and stroke incidences occur annually.
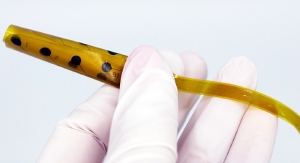
BrainPath is part of a new standardized approach to brain surgery that uses the natural folds of the brain and runs parallel to the fiber tracts as a path to the surgical site. The approach integrates a combination of patented technologies to achieve non-disruptive access, automated tissue removal or clot evacuation, and intraoperative tumor tissue collection and preservation. Using an opening the size of a dime, BrainPath is uniquely designed to gently displace brain tissue as it creates a corridor to the tumor or hemorrhage site. There is no other technology on the market that integrates imaging and intervention and allows for guided atraumatic access within the brain using a trans-sulcal, parafascicular surgical approach. King's College Hospital in London is the first European healthcare institution to be trained on BrainPath.
“The incidence rate of diagnosed brain tumors in the United Kingdom (UK) has increased dramatically over the past four decades and is now at more than 11,000,” said Ranjeev Bhangoo, M.D., neurosurgeon at King’s College Hospital. “Having just acquired this technology at our institution, we can now offer a less invasive surgical option to patients and also provide options for many who might have been told before that their tumor was inoperable.
“This new way to do brain surgery has a growing body of peer-reviewed evidence of improved patient outcomes for both tumor removal and hemorrhagic stroke,” he added. “We’re very excited to be the first in the UK to offer this technology.”
The growing body of clinical data for BrainPath procedures continues to demonstrate its overall safety and performance and that it can be used anywhere in the brain’s white matter where access is a challenge. BrainPath is intended for subcortical access, with specific indications that may include: primary and secondary brain tumors such as glioblastoma, vascular abnormalities that cause intracerebral hemorrhage (ICH) or hemorrhagic stroke and secondary bleeds, and intraventricular tumors or cysts. The technology has been included in 34 peer-reviewed publications, posters and abstracts and more than 50 presentations at national and international neurosurgical conferences.
“Patients who undergo brain tumor surgery routinely face a significant risk of long-term damage to their brain in return for a life-saving procedure,” said David Jenkinson, Ph.D., chief scientific officer at The Brain Tumour Charity in Farnborough, United Kingdom. “Any development which allows neurosurgeons to access and remove tumor tissue with less potential harm to the brain is a welcome step forward. We hope the pioneering use of this technology at King’s College Hospital will lead to its wider introduction around the UK, helping to reduce the harm caused by brain tumors and their treatment – one of the key goals we are working toward at The Brain Tumour Charity.”
NICO partnered with the neurosurgical community and invested significant time and resources in training courses to teach the standardized systems approach and use of the standardized technologies to ensure consistent outcomes. Three annual resident courses teaching the approach in the U.S. are now hosted by the University of Southern California, Emory University Medical School, and Indiana University Health. King’s College Hospital will be the first in the UK to offer a training course.
“Gaining CE Mark approval for BrainPath is our first step in answering increasing global interest in a systems approach to subcortical brain surgery using a standardized approach,” said Jim Pearson, president and CEO of NICO Corporation. “We intend to take a planned approach to global expansion and look forward to making a positive difference in the worldwide brain tumor and stroke incidence rate.”
More than 500 neurosurgeons, residents and fellows are trained on BrainPath and over 4,000 procedures have been completed.
BrainPath is part of a new standardized approach to brain surgery that uses the natural folds of the brain and runs parallel to the fiber tracts as a path to the surgical site. The approach integrates a combination of patented technologies to achieve non-disruptive access, automated tissue removal or clot evacuation, and intraoperative tumor tissue collection and preservation. Using an opening the size of a dime, BrainPath is uniquely designed to gently displace brain tissue as it creates a corridor to the tumor or hemorrhage site. There is no other technology on the market that integrates imaging and intervention and allows for guided atraumatic access within the brain using a trans-sulcal, parafascicular surgical approach. King's College Hospital in London is the first European healthcare institution to be trained on BrainPath.
“The incidence rate of diagnosed brain tumors in the United Kingdom (UK) has increased dramatically over the past four decades and is now at more than 11,000,” said Ranjeev Bhangoo, M.D., neurosurgeon at King’s College Hospital. “Having just acquired this technology at our institution, we can now offer a less invasive surgical option to patients and also provide options for many who might have been told before that their tumor was inoperable.
“This new way to do brain surgery has a growing body of peer-reviewed evidence of improved patient outcomes for both tumor removal and hemorrhagic stroke,” he added. “We’re very excited to be the first in the UK to offer this technology.”
The growing body of clinical data for BrainPath procedures continues to demonstrate its overall safety and performance and that it can be used anywhere in the brain’s white matter where access is a challenge. BrainPath is intended for subcortical access, with specific indications that may include: primary and secondary brain tumors such as glioblastoma, vascular abnormalities that cause intracerebral hemorrhage (ICH) or hemorrhagic stroke and secondary bleeds, and intraventricular tumors or cysts. The technology has been included in 34 peer-reviewed publications, posters and abstracts and more than 50 presentations at national and international neurosurgical conferences.
“Patients who undergo brain tumor surgery routinely face a significant risk of long-term damage to their brain in return for a life-saving procedure,” said David Jenkinson, Ph.D., chief scientific officer at The Brain Tumour Charity in Farnborough, United Kingdom. “Any development which allows neurosurgeons to access and remove tumor tissue with less potential harm to the brain is a welcome step forward. We hope the pioneering use of this technology at King’s College Hospital will lead to its wider introduction around the UK, helping to reduce the harm caused by brain tumors and their treatment – one of the key goals we are working toward at The Brain Tumour Charity.”
NICO partnered with the neurosurgical community and invested significant time and resources in training courses to teach the standardized systems approach and use of the standardized technologies to ensure consistent outcomes. Three annual resident courses teaching the approach in the U.S. are now hosted by the University of Southern California, Emory University Medical School, and Indiana University Health. King’s College Hospital will be the first in the UK to offer a training course.
“Gaining CE Mark approval for BrainPath is our first step in answering increasing global interest in a systems approach to subcortical brain surgery using a standardized approach,” said Jim Pearson, president and CEO of NICO Corporation. “We intend to take a planned approach to global expansion and look forward to making a positive difference in the worldwide brain tumor and stroke incidence rate.”
More than 500 neurosurgeons, residents and fellows are trained on BrainPath and over 4,000 procedures have been completed.