MedInvent LLC03.27.17
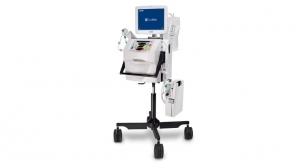
MedInvent LLC has received CE Mark approval for its patented intranasal delivery device, the NasoNeb II Nasal Nebulizer, manufactured, marketed and sold in the United States since 2009. MedInvent also signed its first EU distribution contract with Vedise Hospital in Rome, Italy, to market the NasoNeb System in that country, San Marino and Vatican City. Vedise Hospital has marketed ENT products for over 20 years, serving Italian otolaryngologists.
In addition, MedInvent has obtained ISO 13485 and CMDCAS certification. These certifications, together with CE Marking, enable MedInvent to expand the market for its NasoNeb II Nasal Nebulizer to the member countries of the European Union and to seek regulatory approval in countries around the world.
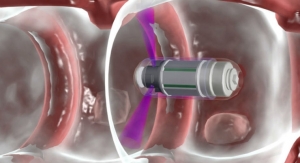
The NasoNeb System has been the delivery device of choice in multiple peer-reviewed trials, where it has demonstrated positive outcomes in chronic sinusitis and allergic rhinitis patients. The System is a controlled intranasal delivery system whereby its unique combination of droplet size, airflow, and fluid volume allows delivery solely to the nasal and paranasal sinus cavities. In addition, the NasoNeb offers broad intranasal drug deposition, high intranasal drug retention, and no pulmonary deposition. Other delivery devices reach a limited area of the nasal cavity, deliver a weak solution, or deliver a portion of the medication to the lungs, which can cause unwanted side effects.
“We look forward to working with European physicians treating patients who suffer with sinus conditions,” said William Flickinger, MedInvent managing partner. “In particular, for those people who have not responded to therapy in the way their physicians would have expected, despite their physician’s best efforts, the NasoNeb System represents a promising new delivery method to ensure that a high concentration of medication is delivered throughout the nasal and paranasal sinus cavities.”
In the United States, the NasoNeb System is available through the member pharmacies of the NasoNeb Pharmacy Network.
MedInvent LLC is a privately-held limited liability corporation with operations in Cleveland, Ohio. MedInvent develops and markets the NasoNeb Family of drug delivery systems for the topical delivery of medication to the nasal and paranasal sinus cavities. MedInvent is a non-diluted company seeking to raise its series A round to fund European Union launch, complete development of next generation products, and undertake clinical trials.
In addition, MedInvent has obtained ISO 13485 and CMDCAS certification. These certifications, together with CE Marking, enable MedInvent to expand the market for its NasoNeb II Nasal Nebulizer to the member countries of the European Union and to seek regulatory approval in countries around the world.
The NasoNeb System has been the delivery device of choice in multiple peer-reviewed trials, where it has demonstrated positive outcomes in chronic sinusitis and allergic rhinitis patients. The System is a controlled intranasal delivery system whereby its unique combination of droplet size, airflow, and fluid volume allows delivery solely to the nasal and paranasal sinus cavities. In addition, the NasoNeb offers broad intranasal drug deposition, high intranasal drug retention, and no pulmonary deposition. Other delivery devices reach a limited area of the nasal cavity, deliver a weak solution, or deliver a portion of the medication to the lungs, which can cause unwanted side effects.
“We look forward to working with European physicians treating patients who suffer with sinus conditions,” said William Flickinger, MedInvent managing partner. “In particular, for those people who have not responded to therapy in the way their physicians would have expected, despite their physician’s best efforts, the NasoNeb System represents a promising new delivery method to ensure that a high concentration of medication is delivered throughout the nasal and paranasal sinus cavities.”
In the United States, the NasoNeb System is available through the member pharmacies of the NasoNeb Pharmacy Network.
MedInvent LLC is a privately-held limited liability corporation with operations in Cleveland, Ohio. MedInvent develops and markets the NasoNeb Family of drug delivery systems for the topical delivery of medication to the nasal and paranasal sinus cavities. MedInvent is a non-diluted company seeking to raise its series A round to fund European Union launch, complete development of next generation products, and undertake clinical trials.