Business Wire05.05.17
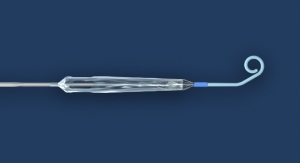
Interscope Inc. announced the receipt of marketing clearance from the FDA for the EndoRotor System in the U.S. to commercialize in gastroenterology and colorectal surgery. Clinical data shows that incomplete resection of colorectal disease is a challenge and rates of disease persistence are as high as 30 percent, leading to an increase in complications. Interscope innovated the first flexible microdebrider for use by medical specialists in the digestive tract to facilitate complete removal of diseased mucosa. Unlike existing instruments, the EndoRotor performs tissue dissection, resection and retrieval in a single step enabling endoscopists to facilitate disease removal without thermal energy.
Interscope CEO, Jeffery Ryan, stated, “This latest milestone marks for our team the culmination of considerable efforts by Interscope and our partners. We thank the FDA and are proud of this achievement as we begin to replicate the success we’ve had in Europe in the US by introducing the first ever GI shaver while continuing to propel advancements in interventional endoscopic patient care.”
Colorectal cancer is the second most common form of cancer accounting for over 150,000 deaths annually. Endoscopic Mucosal Resection (EMR) is a technique for the removal of superficial neoplasms of the GI tract with the goal to avoid disease progression. Although the majority of mucosal neoplasms are non-malignant, risk of malignancy increases with polyp size requiring advanced techniques such as EMR. Studies have shown that the need for enhancing EMR continues to persist. Interscope co-founder and CSO Ramon Franco M.D., a practicing Otolaryngologist stated, “It is vital during invasive procedures to have access to the proper instruments that help to facilitate the work that needs to be done. The wrong instrument can waste time, frustrate the endoscopist and increase the chances of a undesirable outcome. The EndoRotor was designed to make mucosal resection simple, allowing the Endoscopists to concentrate on the other aspects of the procedures.”
Interscope previously announced the receipt of the CE mark in October 2015 to market the EndoRotor in the European Union. Meanwhile the company has established distribution in 10 European countries including direct sales in Germany and the United Kingdom. The EndoRotor System is employed in the management of gastrointestinal mucosal disease at leading centers in Germany, United Kingdom, Netherlands, Switzerland, and Austria. Post-marketing studies in Europe are under way in the Netherlands and United Kingdom with additional studies about to commence in France, Germany, and Italy to demonstrate the EndoRotor patient and physician value proposition.
The U.S. market launch of the EndoRotor System will coincide with Digestive Disease Week international conference, the premier GI event taking place May 6-9 in Chicago, Ill. The EndoRotor System will be exhibited at DDW booth 3911.
Interscope CEO, Jeffery Ryan, stated, “This latest milestone marks for our team the culmination of considerable efforts by Interscope and our partners. We thank the FDA and are proud of this achievement as we begin to replicate the success we’ve had in Europe in the US by introducing the first ever GI shaver while continuing to propel advancements in interventional endoscopic patient care.”
Colorectal cancer is the second most common form of cancer accounting for over 150,000 deaths annually. Endoscopic Mucosal Resection (EMR) is a technique for the removal of superficial neoplasms of the GI tract with the goal to avoid disease progression. Although the majority of mucosal neoplasms are non-malignant, risk of malignancy increases with polyp size requiring advanced techniques such as EMR. Studies have shown that the need for enhancing EMR continues to persist. Interscope co-founder and CSO Ramon Franco M.D., a practicing Otolaryngologist stated, “It is vital during invasive procedures to have access to the proper instruments that help to facilitate the work that needs to be done. The wrong instrument can waste time, frustrate the endoscopist and increase the chances of a undesirable outcome. The EndoRotor was designed to make mucosal resection simple, allowing the Endoscopists to concentrate on the other aspects of the procedures.”
Interscope previously announced the receipt of the CE mark in October 2015 to market the EndoRotor in the European Union. Meanwhile the company has established distribution in 10 European countries including direct sales in Germany and the United Kingdom. The EndoRotor System is employed in the management of gastrointestinal mucosal disease at leading centers in Germany, United Kingdom, Netherlands, Switzerland, and Austria. Post-marketing studies in Europe are under way in the Netherlands and United Kingdom with additional studies about to commence in France, Germany, and Italy to demonstrate the EndoRotor patient and physician value proposition.
The U.S. market launch of the EndoRotor System will coincide with Digestive Disease Week international conference, the premier GI event taking place May 6-9 in Chicago, Ill. The EndoRotor System will be exhibited at DDW booth 3911.