Business Wire08.19.21
The United States Food and Drug Administration (FDA) has granted breakthrough device designation to Abiomed’s Impella ECP expandable percutaneous heart pump. The designation means the FDA will prioritize Impella ECP’s regulatory review processes including design iterations, clinical study protocols and pre-market approval (PMA) application.
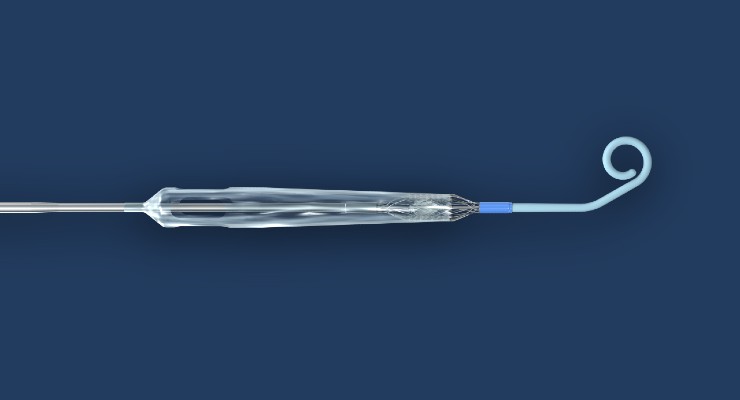
Impella ECP is the smallest heart pump in the world and the first to be compatible with small bore access and closure techniques. It measures 9 French (3 millimeters) in diameter upon insertion and removal from the body. While in the heart, it expands to support the heart’s pumping function, providing flow greater than 3.5 L/min.
The FDA granted breakthrough device designation in part based on positive clinical data from the first 21 Impella ECP patients treated as part of an FDA regulated early feasibility study. In granting the designation, the FDA determined Impella ECP meets the FDA’s stringent requirements for a breakthrough device.

Impella ECP is placed percutaneously into the heart's left ventricle, expands, and supports the heart’s pumping function, providing flow greater than 3.5 L/min. Image courtesy of Business Wire.
“This is yet another validation from the FDA of the clinical benefits of Impella technology and an affirmation of the innovative nature of Impella ECP which, due to its smaller vascular access size, has the potential to provide even safer procedures and be available to more patients who need hemodynamic support for coronary revascularization,” said Chuck Simonton, MD, Abiomed’s chief medical officer.
In the United States, an estimated 440,000 patients are indicated and yet undertreated for high-risk PCI. Impella ECP’s size may enable more physicians to provide critical hemodynamic support to coronary artery disease patients who need it.
The first Impella ECP patient in the world is Robert Matthews, an 80-year-old retired auto worker from Detroit, a father of four, and grandfather of eleven. For more than 20 years, Robert lived with heart disease and endured multiple procedures. In 2020, he was referred to Amir Kaki, MD, an interventional cardiologist and director of mechanical circulatory support at Ascension St. John Hospital in Detroit. Dr. Kaki discovered multiple blockages and poor heart function and identified Robert as an appropriate candidate for a Protected PCI with Impella. Robert became the first patient in the world treated with Impella ECP when Dr. Kaki inserted the heart pump prior to opening blockages and placing stents.
Two days later, Robert returned home, and his family and friends immediately noticed his renewed energy. Today, Robert is grateful for the cutting-edge technology that restored his quality of life.
Impella ECP is the smallest heart pump in the world and the first to be compatible with small bore access and closure techniques. It measures 9 French (3 millimeters) in diameter upon insertion and removal from the body. While in the heart, it expands to support the heart’s pumping function, providing flow greater than 3.5 L/min.
The FDA granted breakthrough device designation in part based on positive clinical data from the first 21 Impella ECP patients treated as part of an FDA regulated early feasibility study. In granting the designation, the FDA determined Impella ECP meets the FDA’s stringent requirements for a breakthrough device.

Impella ECP is placed percutaneously into the heart's left ventricle, expands, and supports the heart’s pumping function, providing flow greater than 3.5 L/min. Image courtesy of Business Wire.
In the United States, an estimated 440,000 patients are indicated and yet undertreated for high-risk PCI. Impella ECP’s size may enable more physicians to provide critical hemodynamic support to coronary artery disease patients who need it.
The first Impella ECP patient in the world is Robert Matthews, an 80-year-old retired auto worker from Detroit, a father of four, and grandfather of eleven. For more than 20 years, Robert lived with heart disease and endured multiple procedures. In 2020, he was referred to Amir Kaki, MD, an interventional cardiologist and director of mechanical circulatory support at Ascension St. John Hospital in Detroit. Dr. Kaki discovered multiple blockages and poor heart function and identified Robert as an appropriate candidate for a Protected PCI with Impella. Robert became the first patient in the world treated with Impella ECP when Dr. Kaki inserted the heart pump prior to opening blockages and placing stents.
Two days later, Robert returned home, and his family and friends immediately noticed his renewed energy. Today, Robert is grateful for the cutting-edge technology that restored his quality of life.