E-QURE Corp. 03.06.17
E-QURE Corp., a developer of advanced wound care treatment devices, has received U.S. Food and Drug Administration (FDA) approval to conduct its pivotal trial for the company's patented Bio-electrical Signal Therapy (BST) device in the treatment of chronic wound care as a staged study, potentially shortening the duration of the trial.
The conditional approval that was granted to E-QURE under an Investigational Device Exemption (IDE) in October 2016 said the company may conduct the safety stage of the trial in one U.S. institution. The company has since requested an increase in the number of institutions in the safety stage and the FDA responded with an approval, increasing the number of U.S. institutions where the first 10 patients will be treated from one to five.
“We are delighted the FDA has granted us the opportunity to shorten the trial period by increasing the number of sites we can use. This may shorten our whole study period by more than six months. We intend to fulfill all FDA requirements and start the trial as soon as we can,” said Ron Weissberg, chairman of E-QURE Corp.
The approval to increase the number of institutions where the first 10 patients can be treated is expected to allow the company to dramatically shorten the safety portion of the trial, resulting in an accelerated timeline to treat all 90 patients planned for inclusion in the study. E-QURE may request approval to expand enrollment in the study to 90 patients following its submission of an IDE supplement which includes a safety profile of the 10 patients who completed 56 days’ treatment with the E-QURE BST device.
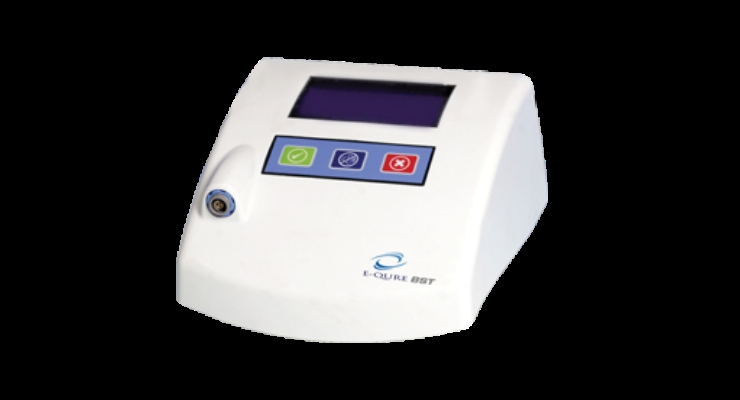
E-QURE Corp.'s patented Bioelectrical Signal Therapy device is the only method in the world to non-invasively close and heal chronic wounds and ulcers. Current methods of chronic wound treatment at best treat or reduce the wound, but none consistently heal or cure it. The BST Device not only cures wounds, it does so faster, at lower cost, and with more convenience to the patient and the healthcare system. The BST Device has received regulatory approval in Europe and Israel, and is expected to launch between the first and third quarters in Israel and Argentina, where approval is pending. E-QURE plans to begin its FDA trial in the first quarter of 2017. The New York, N.Y.-based company’s pipeline includes additional proprietary wound care products in development.
The conditional approval that was granted to E-QURE under an Investigational Device Exemption (IDE) in October 2016 said the company may conduct the safety stage of the trial in one U.S. institution. The company has since requested an increase in the number of institutions in the safety stage and the FDA responded with an approval, increasing the number of U.S. institutions where the first 10 patients will be treated from one to five.
“We are delighted the FDA has granted us the opportunity to shorten the trial period by increasing the number of sites we can use. This may shorten our whole study period by more than six months. We intend to fulfill all FDA requirements and start the trial as soon as we can,” said Ron Weissberg, chairman of E-QURE Corp.
The approval to increase the number of institutions where the first 10 patients can be treated is expected to allow the company to dramatically shorten the safety portion of the trial, resulting in an accelerated timeline to treat all 90 patients planned for inclusion in the study. E-QURE may request approval to expand enrollment in the study to 90 patients following its submission of an IDE supplement which includes a safety profile of the 10 patients who completed 56 days’ treatment with the E-QURE BST device.
E-QURE Corp.'s patented Bioelectrical Signal Therapy device is the only method in the world to non-invasively close and heal chronic wounds and ulcers. Current methods of chronic wound treatment at best treat or reduce the wound, but none consistently heal or cure it. The BST Device not only cures wounds, it does so faster, at lower cost, and with more convenience to the patient and the healthcare system. The BST Device has received regulatory approval in Europe and Israel, and is expected to launch between the first and third quarters in Israel and Argentina, where approval is pending. E-QURE plans to begin its FDA trial in the first quarter of 2017. The New York, N.Y.-based company’s pipeline includes additional proprietary wound care products in development.