Business Wire04.12.17
Acelity, a global advanced wound care company, has launched the V.A.C. VERAFLO CLEANSE CHOICE Dressing, providing clinicians with an adjunctive non-surgical option that may help clean large complex wounds when complete surgical debridement is not possible or appropriate. When used with V.A.C. VERAFLO Therapy, Acelity’s negative pressure wound therapy and instillation (NPWTi-d) system, the dressing may provide rapid cleansing of wounds with the goal of augmenting the healing environment.
Data highlighting the efficacy of the V.A.C. VERAFLO CLEANSE CHOICE Dressing were published in a recent study in the International Wound Journal. In the study of 21 patients, researchers found that using V.A.C. VERAFLO CLEANSE CHOICE Dressing with the V.A.C.ULTA Therapy System helped loosen, solubilize, detach and remove viscous wound exudate such as fibrinous and infectious materials from the wound with and without surgical intervention.i In the study, use of the V.A.C. VERAFLO CLEANSE CHOICE Dressing for full treatment duration and with routine dressing changes reduced the surface area of black non-viable tissue and yellow fibrinous slough to less than ten percent in 18 out of 21 patients.
“The results suggest that the V.A.C. VERAFLO CLEANSE CHOICE Dressing can improve clinical outcomes compared to the current standard of care. The dressing has the potential to revolutionize practice and define a new pathway for treatment,” said Luc Téot, M.D., Ph.D., associate professor of plastic surgery in wound healing and burns at Montpellier University Hospital and lead author of the study. “The broader implications for what this could mean for clinicians and patients—removing wound bioburden at the bedside—could further help facilitate wound healing at a reduced cost, which continues to be a major concern for both patients and healthcare systems overall.”
Chronic and surgical wounds affect more than 10.5 million people in the United States and Europe and can cost more than $50 billion per year,ii,iii,iv yet nearly 30 percent of patients in need of debridement are unable to undergo surgical procedures due to age, pain tolerance or co-morbidities.v The new V.A.C. VERAFLO CLEANSE CHOICE Dressing is designed to help address this patient population by facilitating the disruption and solubilization of materials in the wound bed, cleansing the wound and helping to prepare the wound bed for primary or secondary wound closure.
“We strive to constantly bring forward new advancements in wound care that expand the boundaries of current treatment options,” said Joe Woody, president and CEO of Acelity. “Our leadership position and deep expertise in this field of medicine affords us the opportunity to build an entirely new generation of advanced devices that is both additive to the care continuum and lowers the overall healthcare spend. When considering treatment options for a severe wound, our aim is to provide clinicians and caregivers with greater certainty by offering the most robust and advanced portfolio of solutions that includes industry-leading therapies and the unique service offerings that only Acelity provides.”
The V.A.C. VERAFLO CLEANSE CHOICE Dressing is now available in the United States, Canada, Europe, and Latin America.
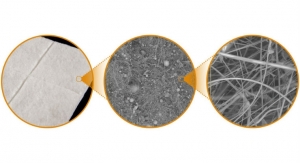
The V.A.C. VERAFLO CLEANSE CHOICE Dressing is made up of three different layers: a specially designed contact layer to provide “mechanical” movement at the wound surface in combination with cyclic delivery and dwell of topical solutions to help remove slough and other bioburden from the wound and two cover layers to provide application options for wounds with varying depths. This three-layer foam design allows for more selective cleaning of compromised tissue and infectious materials compared to traditional NPWT dressings. When used in conjunction with V.A.C. VERAFLO Therapy, the V.A.C. VERAFLO CLEANSE CHOICE Dressing provides a wound cleansing option for clinicians when surgical debridement is not possible or appropriate.
V.A.C. VERAFLO Therapy combines V.A.C. Therapy with automated instillation and removal of topical wound cleansers, and is a therapy offering of the V.A.C.ULTA Therapy System. This therapy allows wounds to be treated with the instillation of topical wound solutions such antimicrobial and antiseptic wound solutions as well as saline to augment favorable wound healing environment and prepare for closure.
Acelity L.P. Inc. and its subsidiaries are a global advanced wound care company that leverages the strengths of Kinetic Concepts Inc. and Systagenix Wound Management Limited. Available in more than 80 countries, the ACELITY product portfolio delivers value through solutions that speed healing and lead the industry in quality, safety and customer experience. Headquartered in San Antonio, Texas, Acelity employs nearly 5,000 people around the world.
References:
i. Téot, L., Boissiere, F. and Fluieraru, S. (2017), Novel foam dressing using negative pressure wound therapy with instillation to remove thick exudate. Int Wound J. doi:10.1111/iwj.12719
ii. Sen CK, Gordillo GM, Roy S, et al. Human Skin Wounds: A Major and Snowballing Threat to Public Health and the Economy. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2009;17(6):763-771.
iii. Ally, A. (2014). Wound care: An answer to the burden of chronic diseases? Retrieved July 07, 2016, from http://medtechviews.eu/article/wound-care-answer-burden-chronic-diseases.
iv. Fife CE, Carter MJ. Wound Care Outcomes and Associated Cost Among Patients Treated in US Outpatient Wound Centers: Data From the US Wound Registry. Wounds. 2012;24(1):10-7.
v. KCI US Conference Surveys ASPS, ACS, AAOS, 2015
Data highlighting the efficacy of the V.A.C. VERAFLO CLEANSE CHOICE Dressing were published in a recent study in the International Wound Journal. In the study of 21 patients, researchers found that using V.A.C. VERAFLO CLEANSE CHOICE Dressing with the V.A.C.ULTA Therapy System helped loosen, solubilize, detach and remove viscous wound exudate such as fibrinous and infectious materials from the wound with and without surgical intervention.i In the study, use of the V.A.C. VERAFLO CLEANSE CHOICE Dressing for full treatment duration and with routine dressing changes reduced the surface area of black non-viable tissue and yellow fibrinous slough to less than ten percent in 18 out of 21 patients.
“The results suggest that the V.A.C. VERAFLO CLEANSE CHOICE Dressing can improve clinical outcomes compared to the current standard of care. The dressing has the potential to revolutionize practice and define a new pathway for treatment,” said Luc Téot, M.D., Ph.D., associate professor of plastic surgery in wound healing and burns at Montpellier University Hospital and lead author of the study. “The broader implications for what this could mean for clinicians and patients—removing wound bioburden at the bedside—could further help facilitate wound healing at a reduced cost, which continues to be a major concern for both patients and healthcare systems overall.”
Chronic and surgical wounds affect more than 10.5 million people in the United States and Europe and can cost more than $50 billion per year,ii,iii,iv yet nearly 30 percent of patients in need of debridement are unable to undergo surgical procedures due to age, pain tolerance or co-morbidities.v The new V.A.C. VERAFLO CLEANSE CHOICE Dressing is designed to help address this patient population by facilitating the disruption and solubilization of materials in the wound bed, cleansing the wound and helping to prepare the wound bed for primary or secondary wound closure.
“We strive to constantly bring forward new advancements in wound care that expand the boundaries of current treatment options,” said Joe Woody, president and CEO of Acelity. “Our leadership position and deep expertise in this field of medicine affords us the opportunity to build an entirely new generation of advanced devices that is both additive to the care continuum and lowers the overall healthcare spend. When considering treatment options for a severe wound, our aim is to provide clinicians and caregivers with greater certainty by offering the most robust and advanced portfolio of solutions that includes industry-leading therapies and the unique service offerings that only Acelity provides.”
The V.A.C. VERAFLO CLEANSE CHOICE Dressing is now available in the United States, Canada, Europe, and Latin America.
The V.A.C. VERAFLO CLEANSE CHOICE Dressing is made up of three different layers: a specially designed contact layer to provide “mechanical” movement at the wound surface in combination with cyclic delivery and dwell of topical solutions to help remove slough and other bioburden from the wound and two cover layers to provide application options for wounds with varying depths. This three-layer foam design allows for more selective cleaning of compromised tissue and infectious materials compared to traditional NPWT dressings. When used in conjunction with V.A.C. VERAFLO Therapy, the V.A.C. VERAFLO CLEANSE CHOICE Dressing provides a wound cleansing option for clinicians when surgical debridement is not possible or appropriate.
V.A.C. VERAFLO Therapy combines V.A.C. Therapy with automated instillation and removal of topical wound cleansers, and is a therapy offering of the V.A.C.ULTA Therapy System. This therapy allows wounds to be treated with the instillation of topical wound solutions such antimicrobial and antiseptic wound solutions as well as saline to augment favorable wound healing environment and prepare for closure.
Acelity L.P. Inc. and its subsidiaries are a global advanced wound care company that leverages the strengths of Kinetic Concepts Inc. and Systagenix Wound Management Limited. Available in more than 80 countries, the ACELITY product portfolio delivers value through solutions that speed healing and lead the industry in quality, safety and customer experience. Headquartered in San Antonio, Texas, Acelity employs nearly 5,000 people around the world.
References:
i. Téot, L., Boissiere, F. and Fluieraru, S. (2017), Novel foam dressing using negative pressure wound therapy with instillation to remove thick exudate. Int Wound J. doi:10.1111/iwj.12719
ii. Sen CK, Gordillo GM, Roy S, et al. Human Skin Wounds: A Major and Snowballing Threat to Public Health and the Economy. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2009;17(6):763-771.
iii. Ally, A. (2014). Wound care: An answer to the burden of chronic diseases? Retrieved July 07, 2016, from http://medtechviews.eu/article/wound-care-answer-burden-chronic-diseases.
iv. Fife CE, Carter MJ. Wound Care Outcomes and Associated Cost Among Patients Treated in US Outpatient Wound Centers: Data From the US Wound Registry. Wounds. 2012;24(1):10-7.
v. KCI US Conference Surveys ASPS, ACS, AAOS, 2015