Content From Life Science Outsourcing Inc.
-
Navigating 2024: 3 Strategies for Strengthening Supply Chain Resilience
As we approach 2024, medical device original equipment manufacturers are at a critical juncture. Read to learn more!Sponsored Content Released on 11.27.2023
-
Considering Scalability in a Manufacturing Partner—A Medtech Makers Q&A
The right service provider can offer an array of capabilities to startups and established device manufacturers.Sponsored Content Released on 10.02.2023
-
OEMs Benefit From Vendor In-House Capabilities & Supply Chain Agility to Dramatically Mitigate Risk
An effective end-to-end outsourcing partner should offer a variety of services to ensure a company's current and future growth needs can and will be met.Sponsored Content Released on 09.18.2023
-
Shifting Medical Device Industry Dynamics Require Contract Sterilization Agility and Novel Solutions
A look at three ways medical device companies successfully navigated industry sterilization challenges and can continue doing so.Sponsored Content Released on 07.12.2023
-
Facility Utilization Offerings for Medtech Startups—A Medtech Makers Q&A
Startups with innovative ideas can have a fully functional space as well as an expert staff in regulatory, packaging, sterilization and more at their disposal.Sponsored Content Released on 06.23.2023
-
Life Science Outsourcing Inc. Acquires J-Pac Medical
Expands into biomaterials and diagnostics end-markets, broadens service offerings, and secures production capabilities on the East Coast and in Costa Rica.Press Releases Released on 01.20.2023
-
Offering Services for Medical Device Incubators—A MedTech Makers Q&A
Helping to get napkin-sketch ideas into the marketplace is a key to continued success.Sponsored Content Released on 06.29.2022
-
Challenges of Contract Medical Device Assembly Post COVID-19 —A Medtech Makers Q&A
The pandemic presented a host of obstacles to overcome, but also provided new best practices based on lessons learned to carry forward.Sponsored Content Released on 12.10.2021

Related Content
-

SCIEX Debuts the ZenoTOF 7600 System
System delivers new capabilities for life science research and biotherapeutic development.Business Wire 09.02.21
-

Foxx Life Sciences Expands with Two New Manufacturing Facilities
The new locations add four additional Single Use Technology Cleanrooms.Foxx Life Sciences 07.30.21
-

Xenter Appoints Four Healthcare Industry Leaders to its Board
Company also closes a $12 million Series A stock offering.Business Wire 07.12.21
-
Surgical

USMI Developing First Robotic System for Cancer Surgery
Stand-alone solution for robotic-assisted surgery will target open, laparoscopic/mini-invasive, endoscopic, thoracoscopic, and trans-oral surgical procedures.Business Wire 07.08.21
-
Diagnostics

How an MDQMS Can Lead to More Efficient and Sustainable Diagnostic Tests
Leveraging technology to improve speed to market without sacrificing quality.Brandon Henning, VP of Product at Greenlight Guru 07.01.21
-

Excelitas Technologies To Acquire PCO AG
PCO’s scientific CMOS camera technologies for high-performance imaging will enhance Excelitas’ photonics portfolio.Excelitas Technologies Corp. 06.29.21
-

Validant Acquires Greenleaf Health
Greenleaf Chief Executive Patrick Ronan named new CEO of Validant.Business Wire 06.14.21
-

Halo Labs Launches Aura CL for Cell Therapy Product Quality
Aura CL is the first system specifically designed to quantify cell aggregates and ID cell vs non-cell contaminants.PR Newswire 06.14.21
-
Electronics
Miniaturizing Linear Motion and Measurement—A Medical Equipment Challenge
A real world example of a successful engineering challenge to develop a smaller, lighter motion control solution.Christian Meier, Senior Product Manager, SCHNEEBERGER 05.14.21
-

Piezo Motion Debuts Piezoelectric Micro-Dosing Pump
Applications that traditionally require two or three separate laboratory devices can now be replaced with a single micro-dosing pump.Michael Barbella, Managing Editor 04.20.21
-
Diagnostics

DiaSorin to Buy Luminex for $1.8B
Provides access to proven Luminex multiplexing technology and molecular testing solutions.Sam Brusco, Associate Editor 04.13.21
-

Access Vascular Secures $20 Million in Series B Financing
Money will be used to generate post-market data on two commercial devices designed to reduce thrombus accumulation and catheter occlusions.Michael Barbella, Managing Editor 03.29.21
-

A Practical Energy Efficiency Solution for Life Science Utilizing Industry 4.0
What relevant and practical data and understanding can I4.0 bring your company in everyday operations?
-
Flywheel Secures $15 Million in Series B Funding
The funding will allow the company to deepen its presence in the life sciences and AI markets.Michael Barbella, Managing Editor 02.19.21
-
Festo is Omnipresent in the Automated Production of Today
Developing individual customer solutions and market-specific products for the Life Science market.
-

Foxx Life Sciences Appoints SVP of Global Sales and Business Development
Mark Robillard brings industry knowledge and experience in all facets of the life science tools industry.Foxx Life Sciences 12.01.20
-

Indago Appoints President and General Counsel
New leader has 15 years of experience in the life sciences industry.Business Wire 11.16.20
-
Chronic Disease

Varian Invests $10M in COTA to Boost Data-Driven Cancer Care
Companies will work together to empower cancer clinics with data analytics and decision support tools.PR Newswire 11.10.20
-
Contract Manufacturing

Freudenberg Medical Completes New Manufacturing Operation
Establishes new facility in Beverly, Massachusetts.Freudenberg Medical 11.10.20
-
Chronic Disease

Pharmaceutical Focus: A Look at Combination Products
Combination products and drug delivery devices aid in patient compliance and a movement toward point-of-care at home.Mark Crawford, Contributing Writer 11.04.20
-
The Lost Year: 2020 Year in Review
Lessons learned from the global coronavirus pandemic.Michael Barbella, Managing Editor 11.04.20
-

BioInformatics Expands Staff and Enhances Capabilities to Meet Client Demand
Top programmers, data analysts recently completed 240 hours of immersive training in market research methodologies.BioInformatics LLC 11.16.17
-
Electronics

Developed for Life Science: Components with Special Features
The automation expert will present a selection of its extremely broad product range at Compamed.SMC 11.12.17
-

ValGenesis Announces Strategic Partnership with VTI Life Sciences
Pair creates comprehensive validation services solution via an integrated paperless validation lifecycle management platform.ValGenesis Inc. 10.04.17
-

ValGenesis Awarded Patent on Paperless Validation Lifecycle Management Process
Company claims patent is the first granted for a paperless validation system.PR Newswire 09.05.17
-

BioLabs North Carolina Announces Cornerstone Partners
GSK; Wexford Science & Technology, LLC; and Wyrick Robbins catalyze establishment of coworking lab for life science startups.BioLabs North Carolina 08.02.17
-
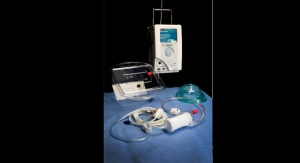
TandemLife Set to Simplify Life Support
Company launches a faster, simpler way to initiate emergent life-support.Business Wire 06.20.17
-

Harvard Bioscience Elects New Board Chairman
Company replacing former chair, who had served for the past four years.Harvard Bioscience Inc. 06.08.17












