Mark Crawford, Contributing Writer11.04.20
As chronic conditions continue to dominate global healthcare, the drug delivery industry is experiencing tremendous growth in new formulations, delivery technologies, and delivery platforms. New advancements in drug delivery systems/combination products are playing a critical role in modernizing the administration of pharmaceuticals and improving the patient experience.
Key growth areas are single use, wearable, and implantable delivery devices, with an emphasis on patient centricity, regulatory compliance, and smart devices. Digitization, interoperability, connectivity, big data, and data security and analytics are other focus areas for combination products that incorporate elements of telemedicine and remote care.
“The drug delivery field is highly competitive for high-volume, well-known drug delivery platforms such as epinephrine or diabetes care,” said Bryan Moris, director of global pre-production quality for Phillips-Medisize, a Molex company that provides innovation, development, and manufacturing solutions for pharmaceutical, diagnostics, and medical device companies. “We see many companies actively vying to be first to market with innovative technologies and combination products for drug delivery.”
Point of care (POC)/homecare is a hot segment in the drug delivery market, driven by the need to control healthcare costs. Safe, easy-to-use combination products are especially in high demand for the homecare market, where instead of a trained healthcare professional delivering the drug to the patient, the patient administers the medication. Also, the real-time transmission of data from patient to physician through POC devices better ensures patient compliance. The FDA is also challenging pharmaceutical companies to bring more generic drugs to market, which will further drive growth in the homecare market.
Much of the innovation in this industry is centered around the patient experience, especially making it easier for patients to adhere to a dosing pattern where they don’t have to be involved at all. Implantable drug delivery devices, for example, combine an active pharmaceutical ingredient (API) with a polymer, which are then implanted in the targeted therapeutic area.
“The benefits of this approach include a steady and predictable elution rate of the API into the body without any patient intervention,” said Kevin Ehlert, segment manager for the Americas for Trelleborg Healthcare & Medical, a Trelleborg, Sweden-based manufacturer of thermoplastic, silicone, and elastomer products for medical, biotech, and pharmaceutical applications. “The patient will receive the desired dose day in, day out. One example of these types of devices is an IUD form of birth control. There are also applications where the device can be planted next to a tumor and a targeted dose delivered directly to the tumor.”
Latest Trends
Cardiovascular is an especially competitive field for device-drug combination products. “Focus segments include drug-coated balloon catheters, drug-eluting bioresorbable scaffolds, and peripheral vascular drug-eluting stents,” noted Ingolf Schult, director of business development and clinical affairs for Hemoteq, a Würselen, Germany-based Freudenberg Medical company that manufactures device-drug combination products and specialty-coated medical devices and components.
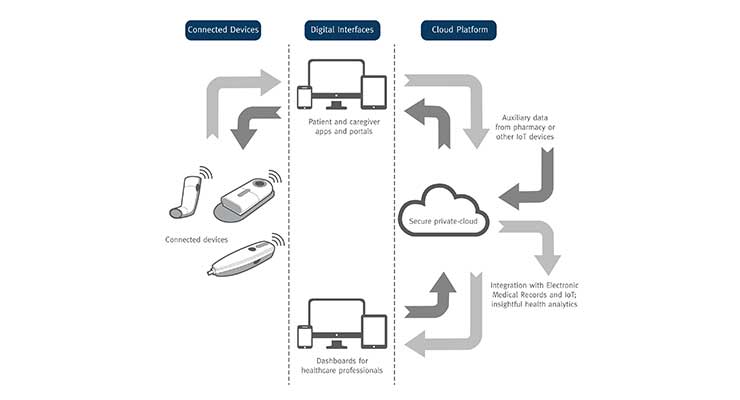
Medical device manufacturers (MDMs) are highly interested in “connected” or “smart” health—the ability of medical devices to share information with a smart device such as a cell phone and then have that data available to the patient and the primary care physician. Combination products with advanced software or user interfaces are becoming more popular, especially as a way to collect data for use in treatment and monitoring of patient compliance.
“We are definitely seeing more interest in combination products as connected devices,” said Todd Macy, director of integrated systems for Gilero, a Morrisville, N.C.-based provider of design, development, and manufacturing services for the medical device industry. “While user needs may vary, the common theme is more about interoperability across the equipment spectrum, increased machine capability, and more data. Data can be used for clinical input or machine learning by way of artificial intelligence. The concept of collecting something like biomarker data that was previously overlooked, building a comprehensive and statistically significant dataset, and then repurposing that data into an algorithm to help with proactive patient care, is here.”
Electronics/connected devices are important drivers in the rapidly growing POC/home healthcare market. Smart devices provide greater patient convenience, better compliance, and real-time transmission of data to their physicians. “This trend was already gaining in popularity prior to the pandemic, but COVID-19 has accelerated demand,” said Kyle Harris, vice president of healthcare for Porex, a Fairburn, Ga.-based provider of porous polymer solutions for the healthcare industry. “With the right drug delivery tools, patients can self-administer drugs safely and in lieu of a hospital or clinic visit.”
“Because of COVID-19, the focus on virtual care and maintaining longer times between hospital visits has strengthened the need for reliable homecare for chronic diseases,” said Asmita Khanolkar, senior director for Cambridge Pharma, a member of the SMC group of companies, headquartered in Somerset, Wis., which provides integrated development, manufacturing, and testing services for drug-device development. “On the drug side, new developments encompass long-acting injectables, intravenous to subcutaneous route of administration, complex formulations, and novel chemistries.”
Considerable research and development is being spent on wearable injector devices, which enable patients to administer large-volume biologics at home, saving frequent trips to the doctor’s office and differentiating products in the rapidly growing, large-molecule space. “Another focus of attention is long-acting injectables and implantables, which not only enable delivery of an API for weeks or months at a time, but also enable lifecycle management and alternative regulatory pathways such as 505(b)(2)s,” said Nick DiFranco, market manager for long-acting drug delivery and combination products for Lubrizol Life Science Health, a Bethlehem, Pa.-based contract development and manufacturing organization (CDMO) that provides drug product formulation and a comprehensive suite of supporting services.
With the focus on injectables for managing chronic diseases, especially through self-administration, there is an increase in the overall use of syringes, particularly disposable syringes, for drug delivery. “We have seen a shift from the use of vials and single-use syringes for a drug product to either prefilled syringes that have a set dose in the syringe that gets fully injected, or to a pen or auto injector,” said Matthew Pasma, testing engineer for DDL, a Minneapolis, Minn.-based provider of medical device package, product, and material testing. “These devices allow the user to set the dose and then inject it much like the traditional vial and single-use syringe method.”
What OEMs Want
Quality, shorter lead times, and reduced costs are always in top demand by OEMs. As the regulatory environment continues to evolve, MDMs are looking to ensure compliance, which means every component in their finished products must perform as intended and protect against potential misuse. This becomes even more important with the trend toward POC devices, where patients are now executing steps on their own, that would have previously been handled by a healthcare professional.
“This is why it is critical for manufacturers to work closely—and early on in the design process—with material suppliers,” said Harris. “Understanding how device materials will behave throughout the administration process allows for a stronger product design and greater protection against human error.”
Patient centricity analysis is essential for minimizing use errors; improving therapeutic safety, efficacy, and clinical outcomes; accelerating product development; and reducing risk from production through commercialization. Patient centricity is another key consideration for developing delivery platforms designed for a variety of challenging formulations. “There is a growing trend toward reusable platforms for devices,” said Jess Pihl, director of quality and regulatory for Phillips-Medisize. “The same device, with slight modifications, can be used/programed to deliver various medications. Using an existing platform to deliver well-known drugs already available reduces time to market and also provides increased value to the patient.”
Another top request by OEMs is a shorter supply chain. They are looking for contract manufacturers that have the ability to reduce time to market and the number of outsourced manufacturing steps by having those various capabilities in-house. This is especially true for drug delivery/combination products, which require cross-disciplinary collaboration among device engineers, pharmaceutical formulators, and contract manufacturers, who bring specialized skills and equipment to the job.
“Companies that are developing the next generation of combination products need access to the facilities and expertise of pharmaceutical manufacturers, especially those with experience in small-batch clinical and commercial manufacturing,” said DiFranco. “Partnerships with pharmaceutical CDMOs enable OEMs to offer complete solutions to their customers, from device design and supply through filling, testing, and packaging of a complete drug product.”
“OEMs want 360-degree expertise in device-drug combination product development, co-development capabilities starting from concept phase, strong IP and in-house technology, fast turnaround during iterative concept improvement, design verification and validation, regulatory expertise, and permanent regulatory focus during project execution, ensuring approvability and marketability of the final product,” added Schult. “In other words, most OEMs want a one-stop-shop for the development and commercialization of their device-drug combination products.”
As drug delivery/combination products become more complex, specialized, and multifunctional, with increased regulatory scrutiny, analytical testing must also evolve to keep up. Testing these unique devices, such as pre-filled syringes, auto-injectors, and pen injectors, must meet highly specific FDA requirements. For instance, testing the physical and mechanical performance of a prefilled syringe measures attributes such as leakage, break loose, extrusion force, and burst resistance, as covered by the ISO 11040 series of standards. “The rapidly growing injectable market has also brought the need for more accurate container closure integrity testing to meet USP-NF<1207> deterministic CCI requirements,” said Pasma.
Technology Advancements
Technology improvements have allowed manufacturers to hold tighter tolerances on their manufacturing processes, resulting in less waste and lower costs. “Many of the dimensions for these parts are held to ± 0.001 mm which is a very tight tolerance,” said Pasma. “This precision is the difference between a part working properly and a device failing in the field.”
“For example, coating or drug reservoir dimensions must be tailored to the amount of drug needed over time to achieve the biological effects required for clinical effectiveness, and to ensure consistent drug release rates as low as double-digit nanogram amounts per day over months, maybe years,” said Schult. “At the same time, safety issues such as local toxicity or foreign body reactions must also be mitigated.”
Controlled drug-delivery options are becoming more diversified—for example, nanoparticle-based drug release, smart polymer-free drug formulations, covalent anchoring and controlled release of drug delivery vehicles (in development), and controlled drug nano-/micro-crystallinity. Other advancements include minimization of drug impurities, additives, matrix components, and particulates.
Mobile applications and the Internet of Things/Industry 4.0 are playing a greater role in device functionality and overall user experience, including patient compliance. Therapeutic solutions are becoming much more integrated—for example, in the treatment of diabetes, “we get closer every day to a true artificial pancreas,” said Ehlert. “There is a sensor that monitors blood glucose level, sends the reading back to a connected device, which then sends feedback to an insulin dosing pump. These systems are becoming increasingly robust and patient-centric. The sensors can be both external to the body as well as implanted. As increasing numbers of applications call for implanted sensors, combining those sensors with an anti-inflammatory steroid will help the body reduce inflammation and increase comfort of the implanted application.”
Both compliance and machine interoperability are attractive benefits of IoT for the drug-delivery devices being developed today. Seldom is the case where only one device is being used to treat a patient, so communication to the outside world, including to other devices, is of great benefit when it comes to drug delivery/combination products. “With enhanced data collection and equipment communication, clinicians are better enabled to make holistic decisions that lead to improved patient outcomes,” said Macy. “Furthermore, through enhanced communication and IoT, the ability to ensure medication compliance is improving patient care and clinician insight.”
The success of IoT depends on advances and learnings in bio-sensors, connectivity, cloud computing, and data analytics. Development of various biomarkers, wireless sensors, and microelectromechanical systems technologies form the sensor and actuator interface. The signal is processed via a microprocessor that actuates a micropump device to deliver the drug. A personal area network or wireless connection to an electronic device like a cell phone or tablet transfers the data via an app. Real-time data transfer to a physician’s web portal is then accomplished via cloud computing for remote monitoring or analysis.
“Radio frequency identification [RFID], coupled with IoT, is playing an increasingly important role within intelligent devices,” said Khanolkar. “Drug type, lot codes, manufacturer, dose, and expiration date can all be encoded in a small RFID component that becomes part of the primary drug container or integrated delivery device, minimizing risk to the patient for misuse and also providing compliance information back to the physician via IoT.”
Additive manufacturing (AM)/3D printing is another Industry 4.0 technology that greatly impacts the drug delivery industry. Companies can prototype parts faster to see if they will work before investing in expensive molds. They can also test multiple designs and materials to see which one is going to work the best for the end user. This helps reduce the overhead costs for making molds to prototype a design. Choosing the right technique requires an understanding of processing advantages/drawbacks and polymer compatibility.
“One of the many challenges with AM is picking the correct material for the application,” said Pasma. “If you use a material that is highly sensitive to temperature, it can become brittle and break after being exposed to some of the extreme requirements that drug delivery devices must face. AM is a great thing for the right application at the right time; however, there are times an injection mold or a machined part is still a better choice for that specific application.”
Some AM techniques produce porous structures with increased surface area relative to densely packed materials. If the material is loaded with a drug, this higher surface area can lead to faster and more complete drug release. “The FDA approved the first 3D-printed drug product, Spiritam [levetiracetam], in 2015,” said DiFranco. “The fast-melt tablets disintegrate in the mouth to rapidly treat certain types of epilepsy. While AM manufacturing continues to generate interest in drug delivery, significant innovation is still happening in traditional drug incorporation techniques, such as hot melt extrusion.”
Additive manufacturing lends itself to IoT technology where the customized product can be 3D-printed remotely as needed, reducing operational costs. 3D printing can also be used for personalized medicine, where the device is made for customized delivery applications. “AM also enables rapid entry into a clinical setting to test a device and/or drug efficacy and effectiveness,” said Khanolkar. “It also allows for early human factors evaluation that can be reflected back into the commercial product, in a faster time frame and at lower cost.”
However, AM still has its manufacturing weak spots—for example, limitations on biomaterials, feature details, cosmetic finishes, and the material performance differences between the AM part and an equivalent injection-molded or a computer numerical control (CNC)-machined part.
Moving Forward
Technology, materials, and designs continue to evolve rapidly for drug delivery/combination products, with a focus on ease of use and reliability. Personalized medicine and targeted therapies include difficult-to-deliver and delicate biotech and biologic drugs. New segments of drug reformulation technologies include nanotechnology, gel-based systems, and emulsion-based systems. Advanced excipient chemistries will enable the development of novel products with unique features, especially for long-acting injectables and implantables.
“The use of novel bioresorbable polymers and solubility-enhancing excipients will usher in the next generation of long-acting injectables,” said DiFranco. “For example, current bioresorbable options are limited and not a great option for many large molecule APIs. Similarly, solubility enhancement is usually reliant on physical/chemical modification of an API or the addition of complex encapsulation techniques. New polymers that address these challenges will enable long-acting delivery of new biologics and improve the efficiency of poorly water-soluble APIs.”
The regulatory aspects of drug delivery/combination products will continue to complicate the approval process.
Due to the complex, multi-disciplinary nature of combination products, the FDA Office of Combination Products (OCP) was created to handle their regulatory filings and coordinate between FDA review centers. OCP assigns each combination product a primary mode of action that determines the lead review center for the product—CDRH (device), CDER (drug), or CBER (biologic). Classification as a drug or biologic requires additional expertise in the investigational new drug application process, including management of phase I-III clinical trials. “Regardless of which lead center is assigned, all three centers are likely to be involved in the approval process through consultations and/or collaborations,” said DiFranco. “Combination product developers should expect to come under scrutiny from multiple FDA agencies as they proceed through the product approval process; having partners with expertise in several regulatory pathways helps tremendously.”
“One of the biggest challenges that our clients face when developing combination products is that typically the company is either a pharmaceutical company or a medical device company first,” stated Kristin Benokraitis, director of quality and operations for Gilero. “This means their quality system, nomenclature, and traditional development pathway may not fully cover all elements of the combination product. In some cases, this either creates a burdensome approach during development, or clients may miss critical elements where the ‘drug’ and the ‘device’ interact. We help our customers translate the device regulatory requirements, when applied to a combination product, to ensure the proper steps are taken during the development process, and the regulatory package includes necessary data and documentation required of a combination product.”
Validation of test methods will face more scrutiny as regulatory agencies increasingly focus on the quality of data, including repeatability and reproducibility. As a result, a shift is occurring toward using deterministic methods for testing. This allows companies to have more statically sound data to analyze to ensure their product is conforming to the standards. "This shift has brought unique challenges in how tests are performed and how they are validated," said Pasma. "New advances in testing equipment and software are always being developed to help make testing more accurate and efficient. Organizations such as ISO and ASTM continue to work to update and refine the standards to improve testing methodologies."
The horizon is bright for innovation in drug delivery, including advances in automation of drug delivery devices coupled with connectivity and smart devices. “Software as a medical device, digital therapeutics, and mobile medical applications will all see substantial growth with various apps for patient use,” said Khanolkar. “Focus will continue on design solutions for delivering challenging biotech and biologics parenteral drugs. Gene therapy delivery devices, organ on a chip, personalized medicine, and targeted drug delivery applications are just some of the other areas of continued interest that will improve therapeutic effects and patient outcomes.”
Mark Crawford is a full-time freelance business and marketing/communications writer based in Madison, Wis. His clients range from startups to global manufacturing leaders. He also writes a variety of feature articles for regional and national publications and is the author of five books.
Key growth areas are single use, wearable, and implantable delivery devices, with an emphasis on patient centricity, regulatory compliance, and smart devices. Digitization, interoperability, connectivity, big data, and data security and analytics are other focus areas for combination products that incorporate elements of telemedicine and remote care.
“The drug delivery field is highly competitive for high-volume, well-known drug delivery platforms such as epinephrine or diabetes care,” said Bryan Moris, director of global pre-production quality for Phillips-Medisize, a Molex company that provides innovation, development, and manufacturing solutions for pharmaceutical, diagnostics, and medical device companies. “We see many companies actively vying to be first to market with innovative technologies and combination products for drug delivery.”
Point of care (POC)/homecare is a hot segment in the drug delivery market, driven by the need to control healthcare costs. Safe, easy-to-use combination products are especially in high demand for the homecare market, where instead of a trained healthcare professional delivering the drug to the patient, the patient administers the medication. Also, the real-time transmission of data from patient to physician through POC devices better ensures patient compliance. The FDA is also challenging pharmaceutical companies to bring more generic drugs to market, which will further drive growth in the homecare market.
Much of the innovation in this industry is centered around the patient experience, especially making it easier for patients to adhere to a dosing pattern where they don’t have to be involved at all. Implantable drug delivery devices, for example, combine an active pharmaceutical ingredient (API) with a polymer, which are then implanted in the targeted therapeutic area.
“The benefits of this approach include a steady and predictable elution rate of the API into the body without any patient intervention,” said Kevin Ehlert, segment manager for the Americas for Trelleborg Healthcare & Medical, a Trelleborg, Sweden-based manufacturer of thermoplastic, silicone, and elastomer products for medical, biotech, and pharmaceutical applications. “The patient will receive the desired dose day in, day out. One example of these types of devices is an IUD form of birth control. There are also applications where the device can be planted next to a tumor and a targeted dose delivered directly to the tumor.”
Latest Trends
Cardiovascular is an especially competitive field for device-drug combination products. “Focus segments include drug-coated balloon catheters, drug-eluting bioresorbable scaffolds, and peripheral vascular drug-eluting stents,” noted Ingolf Schult, director of business development and clinical affairs for Hemoteq, a Würselen, Germany-based Freudenberg Medical company that manufactures device-drug combination products and specialty-coated medical devices and components.
Medical device manufacturers (MDMs) are highly interested in “connected” or “smart” health—the ability of medical devices to share information with a smart device such as a cell phone and then have that data available to the patient and the primary care physician. Combination products with advanced software or user interfaces are becoming more popular, especially as a way to collect data for use in treatment and monitoring of patient compliance.
“We are definitely seeing more interest in combination products as connected devices,” said Todd Macy, director of integrated systems for Gilero, a Morrisville, N.C.-based provider of design, development, and manufacturing services for the medical device industry. “While user needs may vary, the common theme is more about interoperability across the equipment spectrum, increased machine capability, and more data. Data can be used for clinical input or machine learning by way of artificial intelligence. The concept of collecting something like biomarker data that was previously overlooked, building a comprehensive and statistically significant dataset, and then repurposing that data into an algorithm to help with proactive patient care, is here.”
Electronics/connected devices are important drivers in the rapidly growing POC/home healthcare market. Smart devices provide greater patient convenience, better compliance, and real-time transmission of data to their physicians. “This trend was already gaining in popularity prior to the pandemic, but COVID-19 has accelerated demand,” said Kyle Harris, vice president of healthcare for Porex, a Fairburn, Ga.-based provider of porous polymer solutions for the healthcare industry. “With the right drug delivery tools, patients can self-administer drugs safely and in lieu of a hospital or clinic visit.”
“Because of COVID-19, the focus on virtual care and maintaining longer times between hospital visits has strengthened the need for reliable homecare for chronic diseases,” said Asmita Khanolkar, senior director for Cambridge Pharma, a member of the SMC group of companies, headquartered in Somerset, Wis., which provides integrated development, manufacturing, and testing services for drug-device development. “On the drug side, new developments encompass long-acting injectables, intravenous to subcutaneous route of administration, complex formulations, and novel chemistries.”
Considerable research and development is being spent on wearable injector devices, which enable patients to administer large-volume biologics at home, saving frequent trips to the doctor’s office and differentiating products in the rapidly growing, large-molecule space. “Another focus of attention is long-acting injectables and implantables, which not only enable delivery of an API for weeks or months at a time, but also enable lifecycle management and alternative regulatory pathways such as 505(b)(2)s,” said Nick DiFranco, market manager for long-acting drug delivery and combination products for Lubrizol Life Science Health, a Bethlehem, Pa.-based contract development and manufacturing organization (CDMO) that provides drug product formulation and a comprehensive suite of supporting services.
With the focus on injectables for managing chronic diseases, especially through self-administration, there is an increase in the overall use of syringes, particularly disposable syringes, for drug delivery. “We have seen a shift from the use of vials and single-use syringes for a drug product to either prefilled syringes that have a set dose in the syringe that gets fully injected, or to a pen or auto injector,” said Matthew Pasma, testing engineer for DDL, a Minneapolis, Minn.-based provider of medical device package, product, and material testing. “These devices allow the user to set the dose and then inject it much like the traditional vial and single-use syringe method.”
What OEMs Want
Quality, shorter lead times, and reduced costs are always in top demand by OEMs. As the regulatory environment continues to evolve, MDMs are looking to ensure compliance, which means every component in their finished products must perform as intended and protect against potential misuse. This becomes even more important with the trend toward POC devices, where patients are now executing steps on their own, that would have previously been handled by a healthcare professional.
“This is why it is critical for manufacturers to work closely—and early on in the design process—with material suppliers,” said Harris. “Understanding how device materials will behave throughout the administration process allows for a stronger product design and greater protection against human error.”
Patient centricity analysis is essential for minimizing use errors; improving therapeutic safety, efficacy, and clinical outcomes; accelerating product development; and reducing risk from production through commercialization. Patient centricity is another key consideration for developing delivery platforms designed for a variety of challenging formulations. “There is a growing trend toward reusable platforms for devices,” said Jess Pihl, director of quality and regulatory for Phillips-Medisize. “The same device, with slight modifications, can be used/programed to deliver various medications. Using an existing platform to deliver well-known drugs already available reduces time to market and also provides increased value to the patient.”
Another top request by OEMs is a shorter supply chain. They are looking for contract manufacturers that have the ability to reduce time to market and the number of outsourced manufacturing steps by having those various capabilities in-house. This is especially true for drug delivery/combination products, which require cross-disciplinary collaboration among device engineers, pharmaceutical formulators, and contract manufacturers, who bring specialized skills and equipment to the job.
“Companies that are developing the next generation of combination products need access to the facilities and expertise of pharmaceutical manufacturers, especially those with experience in small-batch clinical and commercial manufacturing,” said DiFranco. “Partnerships with pharmaceutical CDMOs enable OEMs to offer complete solutions to their customers, from device design and supply through filling, testing, and packaging of a complete drug product.”
“OEMs want 360-degree expertise in device-drug combination product development, co-development capabilities starting from concept phase, strong IP and in-house technology, fast turnaround during iterative concept improvement, design verification and validation, regulatory expertise, and permanent regulatory focus during project execution, ensuring approvability and marketability of the final product,” added Schult. “In other words, most OEMs want a one-stop-shop for the development and commercialization of their device-drug combination products.”
As drug delivery/combination products become more complex, specialized, and multifunctional, with increased regulatory scrutiny, analytical testing must also evolve to keep up. Testing these unique devices, such as pre-filled syringes, auto-injectors, and pen injectors, must meet highly specific FDA requirements. For instance, testing the physical and mechanical performance of a prefilled syringe measures attributes such as leakage, break loose, extrusion force, and burst resistance, as covered by the ISO 11040 series of standards. “The rapidly growing injectable market has also brought the need for more accurate container closure integrity testing to meet USP-NF<1207> deterministic CCI requirements,” said Pasma.
Technology Advancements
Technology improvements have allowed manufacturers to hold tighter tolerances on their manufacturing processes, resulting in less waste and lower costs. “Many of the dimensions for these parts are held to ± 0.001 mm which is a very tight tolerance,” said Pasma. “This precision is the difference between a part working properly and a device failing in the field.”
“For example, coating or drug reservoir dimensions must be tailored to the amount of drug needed over time to achieve the biological effects required for clinical effectiveness, and to ensure consistent drug release rates as low as double-digit nanogram amounts per day over months, maybe years,” said Schult. “At the same time, safety issues such as local toxicity or foreign body reactions must also be mitigated.”
Controlled drug-delivery options are becoming more diversified—for example, nanoparticle-based drug release, smart polymer-free drug formulations, covalent anchoring and controlled release of drug delivery vehicles (in development), and controlled drug nano-/micro-crystallinity. Other advancements include minimization of drug impurities, additives, matrix components, and particulates.
Mobile applications and the Internet of Things/Industry 4.0 are playing a greater role in device functionality and overall user experience, including patient compliance. Therapeutic solutions are becoming much more integrated—for example, in the treatment of diabetes, “we get closer every day to a true artificial pancreas,” said Ehlert. “There is a sensor that monitors blood glucose level, sends the reading back to a connected device, which then sends feedback to an insulin dosing pump. These systems are becoming increasingly robust and patient-centric. The sensors can be both external to the body as well as implanted. As increasing numbers of applications call for implanted sensors, combining those sensors with an anti-inflammatory steroid will help the body reduce inflammation and increase comfort of the implanted application.”
Both compliance and machine interoperability are attractive benefits of IoT for the drug-delivery devices being developed today. Seldom is the case where only one device is being used to treat a patient, so communication to the outside world, including to other devices, is of great benefit when it comes to drug delivery/combination products. “With enhanced data collection and equipment communication, clinicians are better enabled to make holistic decisions that lead to improved patient outcomes,” said Macy. “Furthermore, through enhanced communication and IoT, the ability to ensure medication compliance is improving patient care and clinician insight.”
The success of IoT depends on advances and learnings in bio-sensors, connectivity, cloud computing, and data analytics. Development of various biomarkers, wireless sensors, and microelectromechanical systems technologies form the sensor and actuator interface. The signal is processed via a microprocessor that actuates a micropump device to deliver the drug. A personal area network or wireless connection to an electronic device like a cell phone or tablet transfers the data via an app. Real-time data transfer to a physician’s web portal is then accomplished via cloud computing for remote monitoring or analysis.
“Radio frequency identification [RFID], coupled with IoT, is playing an increasingly important role within intelligent devices,” said Khanolkar. “Drug type, lot codes, manufacturer, dose, and expiration date can all be encoded in a small RFID component that becomes part of the primary drug container or integrated delivery device, minimizing risk to the patient for misuse and also providing compliance information back to the physician via IoT.”
Additive manufacturing (AM)/3D printing is another Industry 4.0 technology that greatly impacts the drug delivery industry. Companies can prototype parts faster to see if they will work before investing in expensive molds. They can also test multiple designs and materials to see which one is going to work the best for the end user. This helps reduce the overhead costs for making molds to prototype a design. Choosing the right technique requires an understanding of processing advantages/drawbacks and polymer compatibility.
“One of the many challenges with AM is picking the correct material for the application,” said Pasma. “If you use a material that is highly sensitive to temperature, it can become brittle and break after being exposed to some of the extreme requirements that drug delivery devices must face. AM is a great thing for the right application at the right time; however, there are times an injection mold or a machined part is still a better choice for that specific application.”
Some AM techniques produce porous structures with increased surface area relative to densely packed materials. If the material is loaded with a drug, this higher surface area can lead to faster and more complete drug release. “The FDA approved the first 3D-printed drug product, Spiritam [levetiracetam], in 2015,” said DiFranco. “The fast-melt tablets disintegrate in the mouth to rapidly treat certain types of epilepsy. While AM manufacturing continues to generate interest in drug delivery, significant innovation is still happening in traditional drug incorporation techniques, such as hot melt extrusion.”
Additive manufacturing lends itself to IoT technology where the customized product can be 3D-printed remotely as needed, reducing operational costs. 3D printing can also be used for personalized medicine, where the device is made for customized delivery applications. “AM also enables rapid entry into a clinical setting to test a device and/or drug efficacy and effectiveness,” said Khanolkar. “It also allows for early human factors evaluation that can be reflected back into the commercial product, in a faster time frame and at lower cost.”
However, AM still has its manufacturing weak spots—for example, limitations on biomaterials, feature details, cosmetic finishes, and the material performance differences between the AM part and an equivalent injection-molded or a computer numerical control (CNC)-machined part.
Moving Forward
Technology, materials, and designs continue to evolve rapidly for drug delivery/combination products, with a focus on ease of use and reliability. Personalized medicine and targeted therapies include difficult-to-deliver and delicate biotech and biologic drugs. New segments of drug reformulation technologies include nanotechnology, gel-based systems, and emulsion-based systems. Advanced excipient chemistries will enable the development of novel products with unique features, especially for long-acting injectables and implantables.
“The use of novel bioresorbable polymers and solubility-enhancing excipients will usher in the next generation of long-acting injectables,” said DiFranco. “For example, current bioresorbable options are limited and not a great option for many large molecule APIs. Similarly, solubility enhancement is usually reliant on physical/chemical modification of an API or the addition of complex encapsulation techniques. New polymers that address these challenges will enable long-acting delivery of new biologics and improve the efficiency of poorly water-soluble APIs.”
The regulatory aspects of drug delivery/combination products will continue to complicate the approval process.
Due to the complex, multi-disciplinary nature of combination products, the FDA Office of Combination Products (OCP) was created to handle their regulatory filings and coordinate between FDA review centers. OCP assigns each combination product a primary mode of action that determines the lead review center for the product—CDRH (device), CDER (drug), or CBER (biologic). Classification as a drug or biologic requires additional expertise in the investigational new drug application process, including management of phase I-III clinical trials. “Regardless of which lead center is assigned, all three centers are likely to be involved in the approval process through consultations and/or collaborations,” said DiFranco. “Combination product developers should expect to come under scrutiny from multiple FDA agencies as they proceed through the product approval process; having partners with expertise in several regulatory pathways helps tremendously.”
“One of the biggest challenges that our clients face when developing combination products is that typically the company is either a pharmaceutical company or a medical device company first,” stated Kristin Benokraitis, director of quality and operations for Gilero. “This means their quality system, nomenclature, and traditional development pathway may not fully cover all elements of the combination product. In some cases, this either creates a burdensome approach during development, or clients may miss critical elements where the ‘drug’ and the ‘device’ interact. We help our customers translate the device regulatory requirements, when applied to a combination product, to ensure the proper steps are taken during the development process, and the regulatory package includes necessary data and documentation required of a combination product.”
Validation of test methods will face more scrutiny as regulatory agencies increasingly focus on the quality of data, including repeatability and reproducibility. As a result, a shift is occurring toward using deterministic methods for testing. This allows companies to have more statically sound data to analyze to ensure their product is conforming to the standards. "This shift has brought unique challenges in how tests are performed and how they are validated," said Pasma. "New advances in testing equipment and software are always being developed to help make testing more accurate and efficient. Organizations such as ISO and ASTM continue to work to update and refine the standards to improve testing methodologies."
The horizon is bright for innovation in drug delivery, including advances in automation of drug delivery devices coupled with connectivity and smart devices. “Software as a medical device, digital therapeutics, and mobile medical applications will all see substantial growth with various apps for patient use,” said Khanolkar. “Focus will continue on design solutions for delivering challenging biotech and biologics parenteral drugs. Gene therapy delivery devices, organ on a chip, personalized medicine, and targeted drug delivery applications are just some of the other areas of continued interest that will improve therapeutic effects and patient outcomes.”
Mark Crawford is a full-time freelance business and marketing/communications writer based in Madison, Wis. His clients range from startups to global manufacturing leaders. He also writes a variety of feature articles for regional and national publications and is the author of five books.