Christian Meier, Senior Product Manager, SCHNEEBERGER05.14.21
The design engineering team at a dental equipment company was handed a challenging assignment: Develop the industry's first handheld intraoral scanner to perform 3D imaging for dental impressions in the dentist's office. The problem: The scanner's small size required a miniaturized linear motion and measurement system that was not available on the market.
The designers were faced with a question. Should they develop a new linear motion and measurement system internally or seek a supplier to customize its product to meet the scanner's challenging specifications?
The issue haunts design engineers daily across medical, dental, and life science OEMs as their development teams face severe pressure to make their products smaller, lighter, and more accurate.
Collaborative Customization
Product development teams are under severe pressure to make their products smaller, lighter, and more accurate. More and more, they are embracing the practice of collaborative customization with suppliers to fulfill their linear motion and measurement needs.
The concept of suppliers performing custom engineering for original equipment manufacturers (OEMs) is not new. But a more collaborative approach is gaining in popularity as product cycle times and budgets shrink. Collaborative customization on linear motion and integration systems involves a close partnership between the supplier and OEM. By fostering close, highly communicative, and transparent relationships, dramatic breakthroughs in design and cost are possible. Benefits include:
While customization may result in a higher initial price, these benefits typically reduce the total cost of ownership and achieve the OEM's cost targets. It also creates customer-centric products that are not presently available in the market.
Selecting a Collaborative Customization Partner
Not every linear motion supplier is resourced and facilitated to engage in product and system customization. In evaluating vendors, look for the following attributes and capabilities.
A Case in Point

The OEM envisioned a new handheld intraoral scanner that would enable the practitioner to generate in-office 3D impressions of patients' teeth for crowns, bridges, and implants.
A recent project with a dental equipment manufacturer represents a successful application of collaborative customization. The OEM envisioned a new handheld intraoral scanner that would enable the practitioner to generate in-office 3D impressions of patients' teeth for crowns, bridges, and implants. The device required linear motion and measuring systems that would hold the instrument's sensor, fit into a handheld form factor, and move rapidly in extremely small and accurate movements. When finished, the scanner's software processes the images to create a 3D representation of the patient's teeth.
Linear motion and measurement are core technologies in dental and medical equipment, life science instruments, and optical systems. In the past, separate subsystems were required to incorporate these functionalities. Traditional linear motion and measurement systems are large and require additional engineering work to integrate their operation.
The company's engineering team believed that a new technology—the MINISCALE PLUS developed by SCHNEEBERGER—could meet the challenging demands of the new handheld product and installed it in a proof-of-concept prototype.
The MINISCALE PLUS consists of a fixed carriage and rails that move with a maximum acceleration of 300m/s2 back and forth. An integrated scale enables the positioning of a sensor in 0.1-micron resolution. The device maintains exceptionally accurate movement because measurement takes place close to the working process. The MINISCALE PLUS is delivered in a module that drops into the OEM's product—reducing system complexity and dramatically accelerating the development process.
But while the MINISCALE PLUS technology—a key element in the scanner’s functionality—worked in the prototype, it was evident the standard system was too large for the final scanner design.
The dental equipment company then engaged SCHNEEBERGER in a collaborative customization effort to miniaturize the MINISCALE PLUS' components. The two companies' engineering teams worked closely together to achieve the project's daunting design goals: reduce the height of the guide rails by 50 percent and shrink the system's weight from 80 grams to 3 grams.
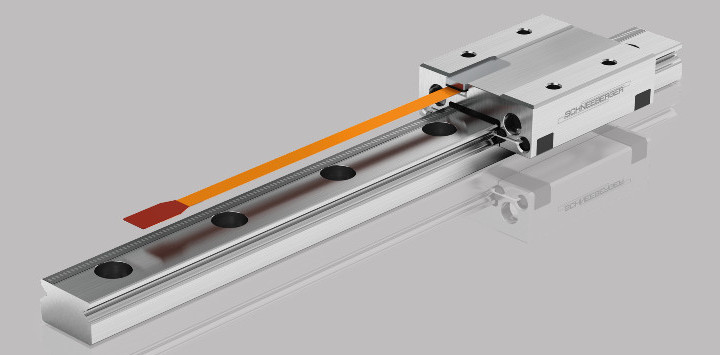
The MINISCALE PLUS needed to have the height of its guide rails reduced by 50 percent and the system's weight cut from 80 grams to 3 grams.
The SCHNEEBERGER engineering and manufacturing teams recognized the standard MINISCALE PLUS system would require a total redesign to meet the OEM's challenging specifications. Most importantly, every aspect of the product's manufacturing process would need to be revised and optimized. This work included raw material selection, pre-machining, hardening, grinding, and assembly. In addition, each component had to be produced using a lean process.
Once completed, the advantages of the collaborative customization project were impressive. They included:
Most importantly, a new dental scanning system was introduced to the market that offered significant competitive advantages. Dentists welcomed its light weight, ease of use, and accuracy, plus the opportunity to improve the patient experience.
Conclusion
As medical and dental equipment, life science instruments, and optical systems decrease in size, innovation in linear motion and measurement systems becomes critical. Collaborative customization has become a viable and cost-effective approach to innovation in component miniaturization. By engaging with suppliers with deep and focused engineering expertise, medical OEMs can accelerate product development, lower assembly costs, and create products that achieve dramatic competitive advantages in the market.
Christian Meier is senior product manager at SCHNEEBERGER Inc., a global leading linear motion company that produces custom stage solutions and linear bearings for the most demanding motion applications in a variety of industries. While earning his degree at ABB Technikerschule, Baden, Meier started his career as a trainee mechanic in a toolmaker company. Over the next 20 years, he gained a wide range of industry expertise in various companies and positions, from international field service engineering to electronic development to training center management to heading assembly operations. He also earned a marketing specialist degree from SGO Business School. Today, at SCHNEEBERGER for seven years, he is senior product manager and head of the company’s MINIRAIL and MINISCALE product lines. You can reach Meier at christian.meier@schneeberger.com or +41 62 918 4251.
The designers were faced with a question. Should they develop a new linear motion and measurement system internally or seek a supplier to customize its product to meet the scanner's challenging specifications?
The issue haunts design engineers daily across medical, dental, and life science OEMs as their development teams face severe pressure to make their products smaller, lighter, and more accurate.
Collaborative Customization
Product development teams are under severe pressure to make their products smaller, lighter, and more accurate. More and more, they are embracing the practice of collaborative customization with suppliers to fulfill their linear motion and measurement needs.
The concept of suppliers performing custom engineering for original equipment manufacturers (OEMs) is not new. But a more collaborative approach is gaining in popularity as product cycle times and budgets shrink. Collaborative customization on linear motion and integration systems involves a close partnership between the supplier and OEM. By fostering close, highly communicative, and transparent relationships, dramatic breakthroughs in design and cost are possible. Benefits include:
- Integration of functions in sub-assemblies results in fewer components
- Designing for manufacture reduces assembly time and costs
- Single sourcing offers one point of accountability for technical support and problem resolution
- Parallel engineering efforts cut product development time
While customization may result in a higher initial price, these benefits typically reduce the total cost of ownership and achieve the OEM's cost targets. It also creates customer-centric products that are not presently available in the market.
Selecting a Collaborative Customization Partner
Not every linear motion supplier is resourced and facilitated to engage in product and system customization. In evaluating vendors, look for the following attributes and capabilities.
- Deep engineering expertise that is focused on linear motion and measurement products and operates with an innovation culture
- Flexible manufacturing systems that can adapt to the new production requirements of a customized product; this includes incorporating lean rules and Industry 4.0 processes that can build high-quality components costs effectively
- Full commitment to reliably meet delivery requirements
- Flexibility to absorb fluctuations in market demand
- Selecting the appropriate customization partner should not be based on price alone
A Case in Point

The OEM envisioned a new handheld intraoral scanner that would enable the practitioner to generate in-office 3D impressions of patients' teeth for crowns, bridges, and implants.
Linear motion and measurement are core technologies in dental and medical equipment, life science instruments, and optical systems. In the past, separate subsystems were required to incorporate these functionalities. Traditional linear motion and measurement systems are large and require additional engineering work to integrate their operation.
The company's engineering team believed that a new technology—the MINISCALE PLUS developed by SCHNEEBERGER—could meet the challenging demands of the new handheld product and installed it in a proof-of-concept prototype.
The MINISCALE PLUS consists of a fixed carriage and rails that move with a maximum acceleration of 300m/s2 back and forth. An integrated scale enables the positioning of a sensor in 0.1-micron resolution. The device maintains exceptionally accurate movement because measurement takes place close to the working process. The MINISCALE PLUS is delivered in a module that drops into the OEM's product—reducing system complexity and dramatically accelerating the development process.
But while the MINISCALE PLUS technology—a key element in the scanner’s functionality—worked in the prototype, it was evident the standard system was too large for the final scanner design.
The dental equipment company then engaged SCHNEEBERGER in a collaborative customization effort to miniaturize the MINISCALE PLUS' components. The two companies' engineering teams worked closely together to achieve the project's daunting design goals: reduce the height of the guide rails by 50 percent and shrink the system's weight from 80 grams to 3 grams.

The MINISCALE PLUS needed to have the height of its guide rails reduced by 50 percent and the system's weight cut from 80 grams to 3 grams.
The SCHNEEBERGER engineering and manufacturing teams recognized the standard MINISCALE PLUS system would require a total redesign to meet the OEM's challenging specifications. Most importantly, every aspect of the product's manufacturing process would need to be revised and optimized. This work included raw material selection, pre-machining, hardening, grinding, and assembly. In addition, each component had to be produced using a lean process.
Once completed, the advantages of the collaborative customization project were impressive. They included:
- Cutting the OEM's product development time by 20 percent over an internally engineered component
- Achieving the customer's cost target
- Lowering the OEM's assembly costs since the integrated MINISCALE PLUS system had fewer components and was plug and play out of the box in the dental scanner
- Providing a single point of accountability and technical support for both the motion and measurement systems
Most importantly, a new dental scanning system was introduced to the market that offered significant competitive advantages. Dentists welcomed its light weight, ease of use, and accuracy, plus the opportunity to improve the patient experience.
Conclusion
As medical and dental equipment, life science instruments, and optical systems decrease in size, innovation in linear motion and measurement systems becomes critical. Collaborative customization has become a viable and cost-effective approach to innovation in component miniaturization. By engaging with suppliers with deep and focused engineering expertise, medical OEMs can accelerate product development, lower assembly costs, and create products that achieve dramatic competitive advantages in the market.
Christian Meier is senior product manager at SCHNEEBERGER Inc., a global leading linear motion company that produces custom stage solutions and linear bearings for the most demanding motion applications in a variety of industries. While earning his degree at ABB Technikerschule, Baden, Meier started his career as a trainee mechanic in a toolmaker company. Over the next 20 years, he gained a wide range of industry expertise in various companies and positions, from international field service engineering to electronic development to training center management to heading assembly operations. He also earned a marketing specialist degree from SGO Business School. Today, at SCHNEEBERGER for seven years, he is senior product manager and head of the company’s MINIRAIL and MINISCALE product lines. You can reach Meier at christian.meier@schneeberger.com or +41 62 918 4251.