Tony Freeman, President, A.S. Freeman Advisors LLC09.06.19
Many medtech supply chain company managers spend time, effort, and worry to obtain their next big customer. Increasingly, their competition for these opportunities are global contract manufacturers whose roots are in the electronics industry. Johnson & Johnson’s Medical Device unit signaled an epic shift toward these multi-billion dollar international suppliers in transferring a large percentage of its manufacturing capability to Jabil Inc. last summer. The Jabil transaction is often unremarked by longtime medical device specialists, yet it should draw focus as the economics point to shifts in the profit margins of the medtech industry. Additionally, there is the possibility that these manufacturing behemoths will, over time, shift the procurement for device components from traditional North American and European suppliers to Asian firms where global contract manufacturers have the local infrastructure to ensure both low prices and good quality.
On Aug. 2, 2018 Johnson & Johnson announced a reorganization of its Medical Devices division. In addition to job cuts, the company stated it had entered into a collaboration with the global contract manufacturer Jabil. The collaboration covered the manufacture of products for J&J’s DePuy Synthes (orthopedic) and Ethicon Endo (minimally invasive surgical devices) business units. J&J expected these actions would save the firm between $600 million and $800 million annually by early 2022. In September 2018, Jabil confirmed rumors that began circulating during the summer—i.e., 14 J&J manufacturing plants in the United States, Germany, and Switzerland would be transferred to Jabil, along with 6,000 J&J employees. In 2019, Jabil reported it had paid $153 million for the facilities, subject to certain adjustments. Though the purchase price sets no records, the deal is the largest transfer of manufacturing capability from an OEM to a supplier in the history of medical device manufacturing. It also marks a major turn in the types of companies that will dominate the medical device supply chain for years to come.
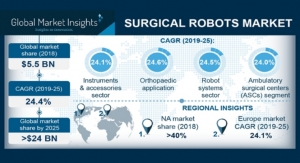
At first glance, $24 billion Jabil is an unlikely manufacturing partner for orthopedic and endoscopic devices. Founded in 1966 in Detroit, Jabil forged its reputation as a contract manufacturer in the electronics industry, serving customers such as Apple, Cisco, and Hewlett-Packard. Fierce competition with other large contract manufacturers like Flextronics drove Jabil to open facilities in 30 countries as well as mastering international supply chains. Jabil’s electronics focus led it to focus on the consumer and office products, telecommunications, and automotive markets. Over time, Jabil brought in a few healthcare companies as customers but their decade-plus relationship with J&J was the cornerstone of their life sciences work. Surprisingly, most of Jabil’s J&J work was for CNC machining and molding rather than electronics.
Transactions require rationale from both the buyer and the seller. Jabil’s reasons for acquiring a significant percentage of J&J Medical Devices’ manufacturing capability has to do with the razor-thin profit margins afforded in the other industries it serves. In 2018, Jabil had a 7.7 percent gross margin and a 3.5 percent operating margin. For those unfamiliar with accounting metrics, these margins are very low and are comparable to the margins of commodity auto parts suppliers. Most medical device supply chain companies earn two to four times more per dollar of revenue than Jabil, a company with 100-plus facilities. With the J&J deal and associated manufacturing contracts, Jabil can expect higher profitability on what it projects will be $1 billion per year in revenue by 2021. Jabil management has projected relevant operating margins to rise to 6 percent or better, still well under medical device averages.
Higher margins are not the only reason global electronic contract manufacturers are intrigued with medical device work. The lifecycle of electronics products is brief. Many consumer devices are on the market for a year or less compared to four to seven years for medical products. Global CMs see the reduced selling, quoting, and setup times helping their bottom line. Jabil chief financial officer Michael Dastoor spoke favorably last fall about the predictable, reliable nature of the J&J work.
J&J’s benefits from the transaction goes beyond immediate cost-savings, as it demonstrates a strategic shift by the world’s second largest medical device manufacturer. A significant portion of a large medtech OEM’s invested capital (the value of its stock and debt, less its cash) is in costly manufacturing facilities. Stepping away from investments in manufacturing should improve J&J’s Medical Devices’ return on invested capital while allowing the company to concentrate on its unique strengths—device design, marketing, sales, and distribution. These core capabilities often require less capital than manufacturing plants. J&J has improved a key investment metric while retaining access to the manufacturing services it requires. Additionally, J&J, like most large legacy OEMs, has notable overhead costs for each employee. Transferring manufacturing overhead to Jabil potentially makes J&J more competitive and profitable. In fairness, this point is speculative; no details of these costs have been publicly released.
Supply chain company managers who have wrestled to meet their OEM customers’ requirements may question a diversified company with modest device experience succeeding in so grand a mission as being J&J’s largest medical device supplier. As a merger and acquisition specialist, I often see the greatest risk of a transaction is not in completing a deal but in the successful integration of an acquisition. To date, there is no evidence that Jabil has stumbled. Dividing the integration of the J&J facilities into three waves, Jabil expects the process to be complete this month (September 2019), only a few weeks beyond the original target date. Additionally, Jabil continues to project annual J&J revenues in the $1 billion range. While it has been a massive effort, the 14 facilities on two continents continue to supply J&J with core products in spite of new ownership.
While it is too early to label the Jabil deal an unqualified success, the transaction is and will continue to be a benchmark event for the supply chain. Supply chain enthusiasts have long argued that any aspect of medical manufacturing can be outsourced. The Jabil deal is a powerful assertion that this view is correct. What is surprising to longtime pundits is that massive transfer of facilities, staff, and programs to a supply chain company went not to a multi-hundred million dollar rollup specializing in medical manufacturing, but to a multi-billion dollar company better known for its work outside the device world. While not putting to rest the strategies that have guided supply chain companies for the last two decades, it challenges the notion that high specialization is the single most important characteristic for success. J&J’s attraction appears to be based on Jabil’s scale and undeniable manufacturing expertise, supplemented by its understanding of J&J and medical devices. Considering J&J went ahead with a deal that encompasses many of its flagship products is a testament to the benefits such large-scale transactions might bring to other OEMs in the future.
Jabil is not unique among global contract manufacturers. Flex has over $2 billion in healthcare revenues, mostly devices. Molex, Celestica, and Plexus also have life science portfolios north of $500 million. Were Jabil not selected as J&J’s outsource partner, these and other firms likely could have filled the role, albeit with significantly less elegance.
A less apparent aspect of the transaction is Jabil’s global sourcing capability. The average medical device sold in developed nations contains content largely manufactured in those countries or a limited number of closely monitored low-cost of manufacture countries. Powerhouses of low-cost manufacturing like China, India, Malaysia, and Vietnam have been shunned due to quality concerns. It is not that Asian manufacturers cannot make components. The challenge has been for OEMs to have the teams in place in those countries to ensure a regular flow of parts manufactured to specification. Jabil and other contract manufacturers have the necessary sourcing teams in place in Asia to guarantee good parts at a lower cost than North American and European suppliers can provide. The trend may start with a few simple pieces but the thin margin ensures global suppliers will have strong incentive to expand their Asian sourcing, to the detriment of traditional component suppliers.
Can established medical device supply chain companies compete with multi-billion dollar global players accustomed to operating on pennies? Strategic change may be necessary for survival. Strategies for thriving among behemoths include superior program management, specialization in complex or technically demanding aspects of devices, and demonstrated project nimbleness. Head-on competition is likely to prove costly and challenging.
Established suppliers should re-examine their strategies in light of the Jabil transaction. J&J’s decision to outsource not only work but facilities and staff will be watched by other large OEMs. OEMs under $2 billion in sales have long outsourced most or all of their manufacturing. The model is not new, but the Jabil deal suggests the scale of the transfers and beneficiaries is changing.
Tony Freeman is president of A.S. Freeman Advisors LLC, an advisor on mergers and acquisitions in the specialty materials and precision manufacturing markets. Based in New York City, A.S. Freeman Advisors also provides corporate strategy, market planning, and valuation improvement consulting services. Tony can be contacted at tfreeman@asfreeman.com.
On Aug. 2, 2018 Johnson & Johnson announced a reorganization of its Medical Devices division. In addition to job cuts, the company stated it had entered into a collaboration with the global contract manufacturer Jabil. The collaboration covered the manufacture of products for J&J’s DePuy Synthes (orthopedic) and Ethicon Endo (minimally invasive surgical devices) business units. J&J expected these actions would save the firm between $600 million and $800 million annually by early 2022. In September 2018, Jabil confirmed rumors that began circulating during the summer—i.e., 14 J&J manufacturing plants in the United States, Germany, and Switzerland would be transferred to Jabil, along with 6,000 J&J employees. In 2019, Jabil reported it had paid $153 million for the facilities, subject to certain adjustments. Though the purchase price sets no records, the deal is the largest transfer of manufacturing capability from an OEM to a supplier in the history of medical device manufacturing. It also marks a major turn in the types of companies that will dominate the medical device supply chain for years to come.
At first glance, $24 billion Jabil is an unlikely manufacturing partner for orthopedic and endoscopic devices. Founded in 1966 in Detroit, Jabil forged its reputation as a contract manufacturer in the electronics industry, serving customers such as Apple, Cisco, and Hewlett-Packard. Fierce competition with other large contract manufacturers like Flextronics drove Jabil to open facilities in 30 countries as well as mastering international supply chains. Jabil’s electronics focus led it to focus on the consumer and office products, telecommunications, and automotive markets. Over time, Jabil brought in a few healthcare companies as customers but their decade-plus relationship with J&J was the cornerstone of their life sciences work. Surprisingly, most of Jabil’s J&J work was for CNC machining and molding rather than electronics.
Transactions require rationale from both the buyer and the seller. Jabil’s reasons for acquiring a significant percentage of J&J Medical Devices’ manufacturing capability has to do with the razor-thin profit margins afforded in the other industries it serves. In 2018, Jabil had a 7.7 percent gross margin and a 3.5 percent operating margin. For those unfamiliar with accounting metrics, these margins are very low and are comparable to the margins of commodity auto parts suppliers. Most medical device supply chain companies earn two to four times more per dollar of revenue than Jabil, a company with 100-plus facilities. With the J&J deal and associated manufacturing contracts, Jabil can expect higher profitability on what it projects will be $1 billion per year in revenue by 2021. Jabil management has projected relevant operating margins to rise to 6 percent or better, still well under medical device averages.
Higher margins are not the only reason global electronic contract manufacturers are intrigued with medical device work. The lifecycle of electronics products is brief. Many consumer devices are on the market for a year or less compared to four to seven years for medical products. Global CMs see the reduced selling, quoting, and setup times helping their bottom line. Jabil chief financial officer Michael Dastoor spoke favorably last fall about the predictable, reliable nature of the J&J work.
J&J’s benefits from the transaction goes beyond immediate cost-savings, as it demonstrates a strategic shift by the world’s second largest medical device manufacturer. A significant portion of a large medtech OEM’s invested capital (the value of its stock and debt, less its cash) is in costly manufacturing facilities. Stepping away from investments in manufacturing should improve J&J’s Medical Devices’ return on invested capital while allowing the company to concentrate on its unique strengths—device design, marketing, sales, and distribution. These core capabilities often require less capital than manufacturing plants. J&J has improved a key investment metric while retaining access to the manufacturing services it requires. Additionally, J&J, like most large legacy OEMs, has notable overhead costs for each employee. Transferring manufacturing overhead to Jabil potentially makes J&J more competitive and profitable. In fairness, this point is speculative; no details of these costs have been publicly released.
Supply chain company managers who have wrestled to meet their OEM customers’ requirements may question a diversified company with modest device experience succeeding in so grand a mission as being J&J’s largest medical device supplier. As a merger and acquisition specialist, I often see the greatest risk of a transaction is not in completing a deal but in the successful integration of an acquisition. To date, there is no evidence that Jabil has stumbled. Dividing the integration of the J&J facilities into three waves, Jabil expects the process to be complete this month (September 2019), only a few weeks beyond the original target date. Additionally, Jabil continues to project annual J&J revenues in the $1 billion range. While it has been a massive effort, the 14 facilities on two continents continue to supply J&J with core products in spite of new ownership.
While it is too early to label the Jabil deal an unqualified success, the transaction is and will continue to be a benchmark event for the supply chain. Supply chain enthusiasts have long argued that any aspect of medical manufacturing can be outsourced. The Jabil deal is a powerful assertion that this view is correct. What is surprising to longtime pundits is that massive transfer of facilities, staff, and programs to a supply chain company went not to a multi-hundred million dollar rollup specializing in medical manufacturing, but to a multi-billion dollar company better known for its work outside the device world. While not putting to rest the strategies that have guided supply chain companies for the last two decades, it challenges the notion that high specialization is the single most important characteristic for success. J&J’s attraction appears to be based on Jabil’s scale and undeniable manufacturing expertise, supplemented by its understanding of J&J and medical devices. Considering J&J went ahead with a deal that encompasses many of its flagship products is a testament to the benefits such large-scale transactions might bring to other OEMs in the future.
Jabil is not unique among global contract manufacturers. Flex has over $2 billion in healthcare revenues, mostly devices. Molex, Celestica, and Plexus also have life science portfolios north of $500 million. Were Jabil not selected as J&J’s outsource partner, these and other firms likely could have filled the role, albeit with significantly less elegance.
A less apparent aspect of the transaction is Jabil’s global sourcing capability. The average medical device sold in developed nations contains content largely manufactured in those countries or a limited number of closely monitored low-cost of manufacture countries. Powerhouses of low-cost manufacturing like China, India, Malaysia, and Vietnam have been shunned due to quality concerns. It is not that Asian manufacturers cannot make components. The challenge has been for OEMs to have the teams in place in those countries to ensure a regular flow of parts manufactured to specification. Jabil and other contract manufacturers have the necessary sourcing teams in place in Asia to guarantee good parts at a lower cost than North American and European suppliers can provide. The trend may start with a few simple pieces but the thin margin ensures global suppliers will have strong incentive to expand their Asian sourcing, to the detriment of traditional component suppliers.
Can established medical device supply chain companies compete with multi-billion dollar global players accustomed to operating on pennies? Strategic change may be necessary for survival. Strategies for thriving among behemoths include superior program management, specialization in complex or technically demanding aspects of devices, and demonstrated project nimbleness. Head-on competition is likely to prove costly and challenging.
Established suppliers should re-examine their strategies in light of the Jabil transaction. J&J’s decision to outsource not only work but facilities and staff will be watched by other large OEMs. OEMs under $2 billion in sales have long outsourced most or all of their manufacturing. The model is not new, but the Jabil deal suggests the scale of the transfers and beneficiaries is changing.
Tony Freeman is president of A.S. Freeman Advisors LLC, an advisor on mergers and acquisitions in the specialty materials and precision manufacturing markets. Based in New York City, A.S. Freeman Advisors also provides corporate strategy, market planning, and valuation improvement consulting services. Tony can be contacted at tfreeman@asfreeman.com.