Maria Shepherd, President and Founder, Medi-Vantage11.26.18
The problem is enormous (no pun intended). According to the CDC, the obesity epidemic is increasing, with estimates placing the prevalence of obesity at 39.8 percent (93.3 million American adults during the study period covering 2015-2016).1 Obesity comorbidities are preventable and include stroke, heart disease, Type 2 diabetes, and certain types of cancer.
Further, it’s not just a problem in the United States. According to an OECD (Organisation for Economic Co-operation and Development) report, obesity rates for adults in 2015 were highest in the U.S., Mexico, New Zealand, and Hungary. The lowest rates were seen in Korea and Japan. OECD forecasts also predict that obesity will continue to increase through 2030, especially in the U.S., Mexico, and England, to 47 percent, 39 percent, and 35 percent of their respective populations (Table 1).2
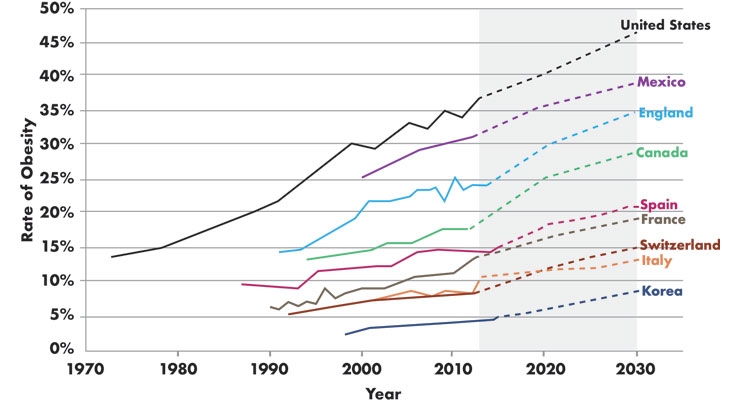
Table 1: Projected Rates of Obesity2
Obesity places a high burden of cost on us all. In a Cornell study, the authors determined the percent of nationwide U.S. medical spend dedicated to treating adult obesity-related illnesses and comorbidities increased from 6.13 percent in 2001 to 7.91 percent in 2015.3 This is a staggering increase of 29 percent. In addition, geography matters in the difference in spend across states.
The Cornell study found that Arizona, California, Florida, New York, and Pennsylvania spent 5 to 6 percent of their total medical disbursements on treating obesity-related disease. North Carolina, Ohio, and Wisconsin spent two times that amount—greater than 12 percent of their healthcare dollars for the treatment of obesity-related disorders.
Why This Is Important
If the OECD forecast is accurate, 47 percent of the U.S. population will be obese by 2030. This is an astonishing statistic—almost half of all Americans. When compared to the significantly smaller number of reported bariatric surgical procedure rates, the situation is mind-boggling. There are many theories as to why the reported number of bariatric surgeries is so low. Between 2011 and 2017, the total reported number of bariatric surgeries increased from 158,000 to 228,000 (Table 2).4 When compared to the anticipated U.S. population that will be considered obese by 2030, the 228,000 bariatric surgeries seems like a miniscule number.
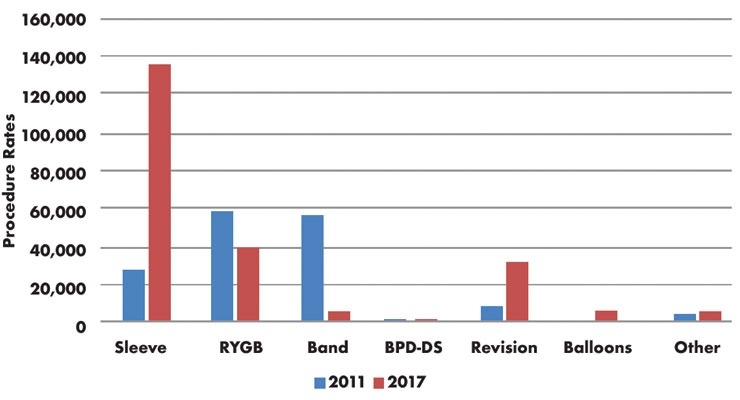
Table 2: Changes in Bariatric Surgery Mix (2011 vs. 2017)4
There are many misconceptions about bariatric surgery. According to the American Society of Metabolic and Bariatric Surgery (ASMBS), the most prevalent fallacy is most patients who have bariatric surgery regain their weight loss.5 Longitudinal studies indicate that successful weight loss, over the long term, is achievable for the majority of bariatric surgery patients, as compared to the experience most of those patients report with non-surgical therapies, such as dieting.
Other misconceptions5 the ASMBS disputes include:
Bariatric aspiration therapy utilizes a device similar to a feeding tube—the gold standard for patients who cannot ingest food orally—but for the reverse process. Instead of delivering food to the stomach, this port aspirates food using a pump that attaches an external valve to the outside of the patient’s stomach. This enables the patient to withdraw up to 30 percent of their most recent meal and dispose of it before it is converted to calories during digestion. This device has been commercialized as AspireAssist from Aspire Bariatrics.
Critics of the device abound, with some calling aspiration therapy “medically assisted bulimia.”6 In a small clinical study (n=171) over a 12-month period, patients using the AspireAssist in combination with lifestyle counseling lost approximately 18.6 percent of their body weight. Patients in the control group received only lifestyle counseling and lost 5.9 percent of their body weight (Table 3). The researchers determined that patients in the AspireAssist cohort were not eating more to make up for the lower number of calories and they were not developing eating disorders. AspireAssist requires patients to eat carefully and to chew fully. The device is reversible and can be removed when the patient feels comfortable. At every meal, the patient can decide whether to use the device or not.

Table 3: Data on Weight Loss During AspireAssist Trial (n=171)
The Medi-Vantage Perspective
This device has everything we seek in truly innovative new medical devices. It is simple to implant, using well established medical technology. It is far less invasive than bariatric surgery. It is safe and effective. It has few side effects if a patient eats too much. It is simple for patients to use and is reversible should the patient decide to discontinue therapy.
In almost every strategy research project we manage, when we look at adjacent technologies, we often find existing medical technologies (like the feeding tube) can be adapted to new uses. Current medical technology represents a broad opportunity to develop new medtech product strategies. What is inside your product portfolio that could be repurposed to address unmet clinical needs?
References
Maria Shepherd has more than 20 years of leadership experience in medical device/life science marketing in small startups and top-tier companies. After her industry career, including her role as vice president of marketing for Oridion Medical, where she boosted the company valuation prior to its acquisition by Covidien/Medtronic, director of marketing for Philips Medical, and senior management roles at Boston Scientific Corp., she founded Medi-Vantage. Medi-Vantage provides marketing, business strategy, and innovation research for the medical device, diagnostic, and digital health industries. The firm quantitatively and qualitatively sizes and segments opportunities, evaluates new technologies, provides marketing services, and assesses prospective acquisitions. Shepherd has taught marketing and product development courses and is a member of the Aligo Medtech Investment Committee (www.aligo.com). She can be reached at 855-343-3100. Visit her website at www.medi-vantage.com.
Further, it’s not just a problem in the United States. According to an OECD (Organisation for Economic Co-operation and Development) report, obesity rates for adults in 2015 were highest in the U.S., Mexico, New Zealand, and Hungary. The lowest rates were seen in Korea and Japan. OECD forecasts also predict that obesity will continue to increase through 2030, especially in the U.S., Mexico, and England, to 47 percent, 39 percent, and 35 percent of their respective populations (Table 1).2

Table 1: Projected Rates of Obesity2
Obesity places a high burden of cost on us all. In a Cornell study, the authors determined the percent of nationwide U.S. medical spend dedicated to treating adult obesity-related illnesses and comorbidities increased from 6.13 percent in 2001 to 7.91 percent in 2015.3 This is a staggering increase of 29 percent. In addition, geography matters in the difference in spend across states.
The Cornell study found that Arizona, California, Florida, New York, and Pennsylvania spent 5 to 6 percent of their total medical disbursements on treating obesity-related disease. North Carolina, Ohio, and Wisconsin spent two times that amount—greater than 12 percent of their healthcare dollars for the treatment of obesity-related disorders.
Why This Is Important
If the OECD forecast is accurate, 47 percent of the U.S. population will be obese by 2030. This is an astonishing statistic—almost half of all Americans. When compared to the significantly smaller number of reported bariatric surgical procedure rates, the situation is mind-boggling. There are many theories as to why the reported number of bariatric surgeries is so low. Between 2011 and 2017, the total reported number of bariatric surgeries increased from 158,000 to 228,000 (Table 2).4 When compared to the anticipated U.S. population that will be considered obese by 2030, the 228,000 bariatric surgeries seems like a miniscule number.

Table 2: Changes in Bariatric Surgery Mix (2011 vs. 2017)4
There are many misconceptions about bariatric surgery. According to the American Society of Metabolic and Bariatric Surgery (ASMBS), the most prevalent fallacy is most patients who have bariatric surgery regain their weight loss.5 Longitudinal studies indicate that successful weight loss, over the long term, is achievable for the majority of bariatric surgery patients, as compared to the experience most of those patients report with non-surgical therapies, such as dieting.
Other misconceptions5 the ASMBS disputes include:
- The probability of dying from bariatric surgery is higher than the chance of dying from obesity.
- Bariatric surgery is unnecessary. To lose weight, obese patients need to go on a diet and exercise more.
- Bariatric surgery increases the risk of suicide and/or alcoholism.
- Obese patients have health issues triggered by vitamin and/or mineral deficiencies.
- Obesity should be treated as an addiction, in the same way as alcoholism or drug dependency.
Bariatric aspiration therapy utilizes a device similar to a feeding tube—the gold standard for patients who cannot ingest food orally—but for the reverse process. Instead of delivering food to the stomach, this port aspirates food using a pump that attaches an external valve to the outside of the patient’s stomach. This enables the patient to withdraw up to 30 percent of their most recent meal and dispose of it before it is converted to calories during digestion. This device has been commercialized as AspireAssist from Aspire Bariatrics.
Critics of the device abound, with some calling aspiration therapy “medically assisted bulimia.”6 In a small clinical study (n=171) over a 12-month period, patients using the AspireAssist in combination with lifestyle counseling lost approximately 18.6 percent of their body weight. Patients in the control group received only lifestyle counseling and lost 5.9 percent of their body weight (Table 3). The researchers determined that patients in the AspireAssist cohort were not eating more to make up for the lower number of calories and they were not developing eating disorders. AspireAssist requires patients to eat carefully and to chew fully. The device is reversible and can be removed when the patient feels comfortable. At every meal, the patient can decide whether to use the device or not.

Table 3: Data on Weight Loss During AspireAssist Trial (n=171)
The Medi-Vantage Perspective
This device has everything we seek in truly innovative new medical devices. It is simple to implant, using well established medical technology. It is far less invasive than bariatric surgery. It is safe and effective. It has few side effects if a patient eats too much. It is simple for patients to use and is reversible should the patient decide to discontinue therapy.
In almost every strategy research project we manage, when we look at adjacent technologies, we often find existing medical technologies (like the feeding tube) can be adapted to new uses. Current medical technology represents a broad opportunity to develop new medtech product strategies. What is inside your product portfolio that could be repurposed to address unmet clinical needs?
References
- http://bit.ly/mpo181201
- http://bit.ly/mpo181202
- http://bit.ly/mpo181203
- http://bit.ly/mpo181204
- http://bit.ly/mpo181205
- http://bit.ly/mpo181206
Maria Shepherd has more than 20 years of leadership experience in medical device/life science marketing in small startups and top-tier companies. After her industry career, including her role as vice president of marketing for Oridion Medical, where she boosted the company valuation prior to its acquisition by Covidien/Medtronic, director of marketing for Philips Medical, and senior management roles at Boston Scientific Corp., she founded Medi-Vantage. Medi-Vantage provides marketing, business strategy, and innovation research for the medical device, diagnostic, and digital health industries. The firm quantitatively and qualitatively sizes and segments opportunities, evaluates new technologies, provides marketing services, and assesses prospective acquisitions. Shepherd has taught marketing and product development courses and is a member of the Aligo Medtech Investment Committee (www.aligo.com). She can be reached at 855-343-3100. Visit her website at www.medi-vantage.com.


















