Maria Shepherd, President and Founder, Medi-Vantage10.16.18
The number of conferences focused on finding funding in the life sciences and medtech space continues to increase. Two of them—the RESI meeting (Redefining Early Stage Investments, held in Boston, Mass., on Sept. 6) and the McDermott Life Sciences Dealmaking Symposium (to be held Oct. 10, 2018 in Cambridge, Mass.)—feature early-stage investors, fundraising CEOs, entrepreneurs, strategic partners, and service providers who attend these meetings to learn about each other and discover best practices for strategy and transactions.
Why This Is Important
One big takeaway from the RESI meeting was the sheer number of incubators and accelerators focused on life sciences that have emerged over the past five years. These programs are technology engines increasing the success rates of startups. According to one website,1 there are 227 accelerators in the United States and 243 entrepreneurship programs at universities across America that self-identify as startup accelerators with dedicated centers for entrepreneurship (Chart 1).
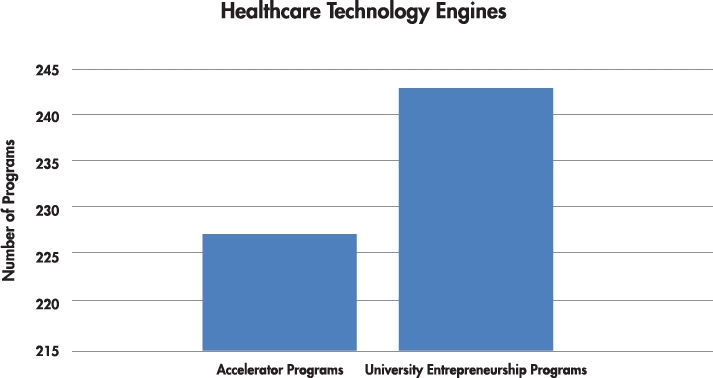
Chart 1: Number of Accelerators and University Programs in the U.S.1
The help provided by these programs is desperately needed. While startups in healthcare are among those with the lowest failure rates compared to other industry sectors, the harsh reality is that only an estimated 56 percent are still operating after four years.2 Incubators, accelerators, and strong academic institutions have service providers able to assist in the design, development, and clinical testing of a device, as well as market assessment and analysis. Further, they can connect startups to groups of experienced entrepreneurs willing to take new technologies to and beyond the point of commercialization.
Who Are the Investors?
Ambitious entrepreneurs who wish to make their startups successful need to understand who the partners and investors are, and what they want. Strategic partners at the RESI meeting stated that there are two types of startup companies—those that have a perfect strategic fit with their divisions (the smaller group), and those who are forging ahead with new devices that may have high value, but a poor strategic fit (the larger group). At the end of the day, a strategic partner has to be able to sell the device through its business units, so strategic fit is important to success. In Chart 2, investor types that routinely (or not so routinely) invest in startups are presented. In the same article,3 Life Sciences Nation founder and CEO Dennis Ford presents several key criteria that investors use to evaluate early-stage opportunities. The message? Tailor the presentation you make to investors by type to specifically address their investment goals.
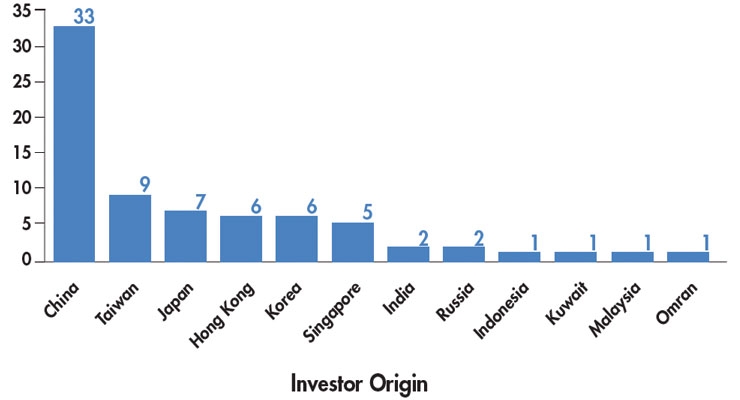
Chart 2: Percentages of Investor Types Seeking Global Opportunities3
Original source: LSN Investor Platform, as of October 1, 2015
Our Medtech Ecosystem Is Changing
The RESI event was truly a global meeting. Most impressive was the number of Asian investors in attendance who were seeking opportunities (Chart 3). Asian medtech/life sciences investors are back in the game and attended the RESI meeting to assess investments or technology licensing outside of Asia. Asian investors know that markets for life science companies have high potential for a profitable exit. U.S. companies are especially attractive because of the strong ecosystem for innovation support, as illustrated in Chart 1.
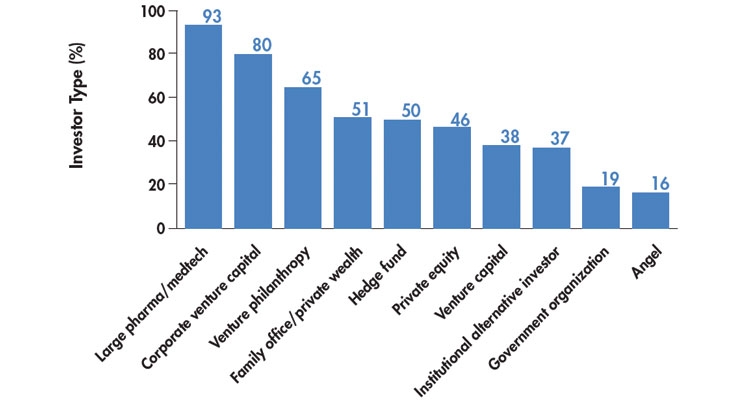
Chart 3. Number of Asian Investors Reviewing Western Healthcare Opportunities3
Original source: LSN Investor Platform, as of October 1, 2015
In addition, there are a large number of interested and well-funded investors in Asia and a relatively low number of life science companies located there, increasing the demand for overseas assets. According to the article,3 some Asian investors may solely be motivated to invest to obtain the right to distribute in their local geographies, instead of acquiring sole global ownership of the asset.
The Medi-Vantage Perspective
As evidenced by attendance at the RESI meeting, the number of Asian investors has grown as compared to those presented in the referenced article, written in 2015.3 Accepting investment in return for local geographic distribution rights is attractive to cash-starved startups, since Asian investors often have robust relationships with manufacturers and distribution channels in their local geography and can become a resource for taking market share in Asia for your medtech product. The conundrum is that strategic partners like Medtronic or Boston Scientific want worldwide rights to a product they acquire. Be sure your contracts for local geographic distribution rights contain an escape clause that supports your global strategy.
References
Maria Shepherd has more than 20 years of leadership experience in medical device/life science marketing in small startups and top-tier companies. After her industry career, including her role as vice president of marketing for Oridion Medical, where she boosted the company valuation prior to its acquisition by Covidien/Medtronic, director of marketing for Philips Medical, and senior management roles at Boston Scientific Inc., she founded Medi-Vantage. Medi-Vantage provides marketing, business strategy, and innovation research for the medical device, diagnostic, and digital health industries. The firm quantitatively and qualitatively sizes and segments opportunities, evaluates new technologies, provides marketing services, and assesses prospective acquisitions. Shepherd has taught marketing and product development courses and is a member of the Aligo Medtech Investment Committee (www.aligo.com). She can be reached at 855-343-3100. Visit her website at www.medi-vantage.com.
Why This Is Important
One big takeaway from the RESI meeting was the sheer number of incubators and accelerators focused on life sciences that have emerged over the past five years. These programs are technology engines increasing the success rates of startups. According to one website,1 there are 227 accelerators in the United States and 243 entrepreneurship programs at universities across America that self-identify as startup accelerators with dedicated centers for entrepreneurship (Chart 1).

Chart 1: Number of Accelerators and University Programs in the U.S.1
The help provided by these programs is desperately needed. While startups in healthcare are among those with the lowest failure rates compared to other industry sectors, the harsh reality is that only an estimated 56 percent are still operating after four years.2 Incubators, accelerators, and strong academic institutions have service providers able to assist in the design, development, and clinical testing of a device, as well as market assessment and analysis. Further, they can connect startups to groups of experienced entrepreneurs willing to take new technologies to and beyond the point of commercialization.
Who Are the Investors?
Ambitious entrepreneurs who wish to make their startups successful need to understand who the partners and investors are, and what they want. Strategic partners at the RESI meeting stated that there are two types of startup companies—those that have a perfect strategic fit with their divisions (the smaller group), and those who are forging ahead with new devices that may have high value, but a poor strategic fit (the larger group). At the end of the day, a strategic partner has to be able to sell the device through its business units, so strategic fit is important to success. In Chart 2, investor types that routinely (or not so routinely) invest in startups are presented. In the same article,3 Life Sciences Nation founder and CEO Dennis Ford presents several key criteria that investors use to evaluate early-stage opportunities. The message? Tailor the presentation you make to investors by type to specifically address their investment goals.

Chart 2: Percentages of Investor Types Seeking Global Opportunities3
Original source: LSN Investor Platform, as of October 1, 2015
Our Medtech Ecosystem Is Changing
The RESI event was truly a global meeting. Most impressive was the number of Asian investors in attendance who were seeking opportunities (Chart 3). Asian medtech/life sciences investors are back in the game and attended the RESI meeting to assess investments or technology licensing outside of Asia. Asian investors know that markets for life science companies have high potential for a profitable exit. U.S. companies are especially attractive because of the strong ecosystem for innovation support, as illustrated in Chart 1.

Chart 3. Number of Asian Investors Reviewing Western Healthcare Opportunities3
Original source: LSN Investor Platform, as of October 1, 2015
In addition, there are a large number of interested and well-funded investors in Asia and a relatively low number of life science companies located there, increasing the demand for overseas assets. According to the article,3 some Asian investors may solely be motivated to invest to obtain the right to distribute in their local geographies, instead of acquiring sole global ownership of the asset.
The Medi-Vantage Perspective
As evidenced by attendance at the RESI meeting, the number of Asian investors has grown as compared to those presented in the referenced article, written in 2015.3 Accepting investment in return for local geographic distribution rights is attractive to cash-starved startups, since Asian investors often have robust relationships with manufacturers and distribution channels in their local geography and can become a resource for taking market share in Asia for your medtech product. The conundrum is that strategic partners like Medtronic or Boston Scientific want worldwide rights to a product they acquire. Be sure your contracts for local geographic distribution rights contain an escape clause that supports your global strategy.
References
Maria Shepherd has more than 20 years of leadership experience in medical device/life science marketing in small startups and top-tier companies. After her industry career, including her role as vice president of marketing for Oridion Medical, where she boosted the company valuation prior to its acquisition by Covidien/Medtronic, director of marketing for Philips Medical, and senior management roles at Boston Scientific Inc., she founded Medi-Vantage. Medi-Vantage provides marketing, business strategy, and innovation research for the medical device, diagnostic, and digital health industries. The firm quantitatively and qualitatively sizes and segments opportunities, evaluates new technologies, provides marketing services, and assesses prospective acquisitions. Shepherd has taught marketing and product development courses and is a member of the Aligo Medtech Investment Committee (www.aligo.com). She can be reached at 855-343-3100. Visit her website at www.medi-vantage.com.