Maria Shepherd, President and Founder, Medi-Vantage06.04.18
Who could have predicted all the changes we have seen in medtech in the 15 years since 2003? In 2003, pacemakers were only used in cardiology. Since then, modifications of early pacemaker technology have erupted into the neurostimulation market. In May 2018 at the Neurotech Partnering Conference, it was estimated the neurostimulation sector would reach $13.4 billion by 2022.1
Additionally, in 2003, fee-for-service was the standard of care and almost no one knew about Accountable Care Organizations. Price premiums for incremental changes in medical devices were still the norm, and most hospitals had never heard of a Value Analysis Committee. The rise of digital health technology was a gleam in the eye of an entrepreneur, and most of us still used our Blackberries for placing a call and an occasional check of email.
Why This Is Important
Despite all the progress we have made, medical device technology is still in the early innovation stage. The next 15 years are going to bring even more change to the practice of providing healthcare and our role in improving healthcare with new medical technology. The changes in innovation are expected to drive growth in the global medical device market from 2017’s $403 billion to $522 billion in 2022,2 representing a healthy CAGR of 6.7 percent. Consistent global figures for the worldwide medical device market in 2003 are difficult to find, but back then, the standard measure was to multiply the U.S. market for medical devices by two to estimate the size of the global market. Using this calculation yields a 2003 global market for medical devices of $204.2 billion3 (Table 1).
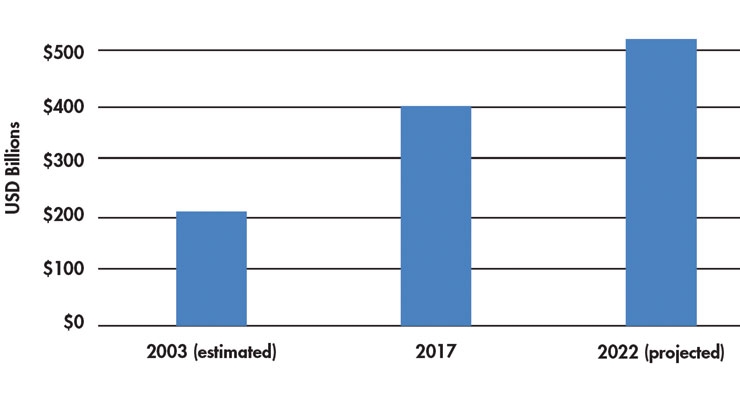
Table 1: Worldwide growth in the medical device market2,3
Price Strategies Have Become Critical in Medtech
Fifteen years ago, an incremental feature improvement in a medtech product could result in a 5 percent price increase with no questions asked by healthcare. Today, those days are long since over. Further, bringing new medical devices to market is much more expensive. In an analysis of the estimated costs of bringing a PMA product to market, a 2010 Stanford study4 showed the cost of regulatory approval is high and also cited the cost of a PMA in the 1990s to be between $30 to $40 million (Table 2). This means price strategy, from simple qualitative research to a conjoint analysis, while expensive, can be a critical component of a new product strategy to ensure medical device companies earn every penny a new innovative technology deserves.
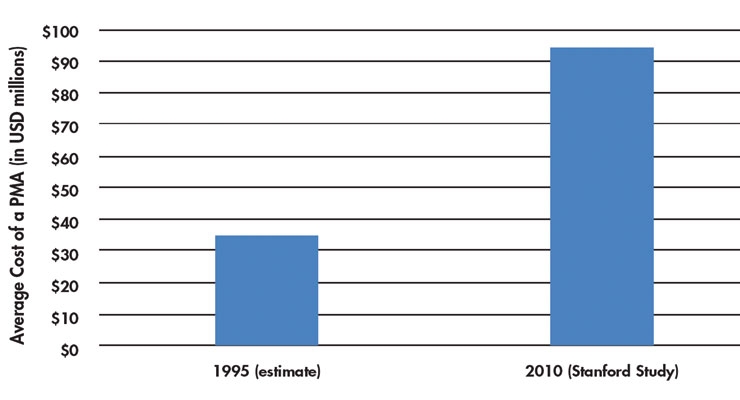
Table 2: Changes in the cost of a PMA4
Mobile Health Apps Didn’t Exist in 2003
The global mHealth market was forecasted to reach $21.5 billion by 20185 (Table 3). mHealth apps have improved in the last five years with the increasing population of tablet and smartphone users, even in the elderly segment (>65 years old)—great news for our digital health clients! Other products widely used are wearable devices, such as Fitbits, glucose sensors, and digital blood pressure monitors.
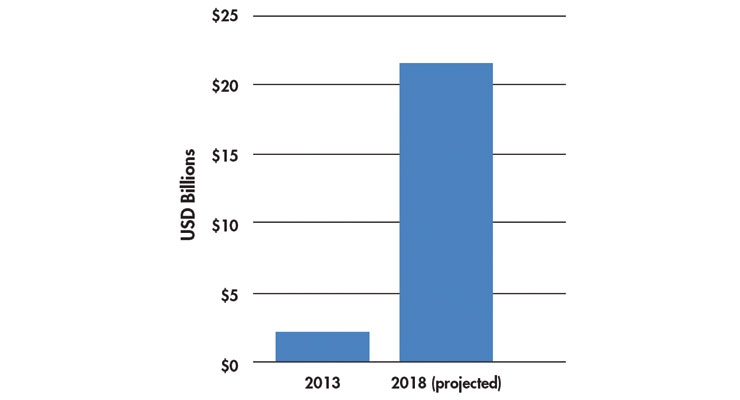
Table 3: Global mHealth growth5
Expect to see some of these apps on your phone to monitor changes in physiology. Further, look for broader adoption by patients and healthcare providers. Patients get the benefit of easier communication with their healthcare provider and more information on symptoms and medical conditions, which decreases anxiety and reduces the communications burden on healthcare providers. Healthcare providers can expect to see lower patient utilization of services (such as unnecessary emergency room visits) and a focus on improving outcomes and preventive medicine.
The Medi-Vantage Perspective
In almost every strategy research project we manage, we start with a review of adjacent technologies in the consumer markets. This review helps inform our strategy research, which often involves understanding how to integrate new technology, such as artificial intelligence (AI), into client product strategies. For example, at the World Medical Innovation Forum, held in April 2018, the majority of the Disruptive Dozen AI-based technologies6 that will have the greatest impact on healthcare in the next decade can all point to consumer-based technological advances that provided these platforms. There is no need to re-invent the wheel; there are plenty of good technological advances in development that can be modified to assist in medtech. Consider that in your product development strategy over the next 15 years.
References
Maria Shepherd has more than 20 years of leadership experience in medical device/life-science marketing in small startups and top-tier companies. After her industry career, including her role as VP of marketing for Oridion Medical where she boosted the company valuation prior to its acquisition by Covidien/Medtronic, director of marketing for Philips Medical, and senior management roles at Boston Scientific Corp., she founded Medi-Vantage. Medi-Vantage provides marketing and business strategy as well as innovation research for the medical device industry. The firm quantitatively and qualitatively sizes and segments opportunities, evaluates new technologies, provides marketing services, and assesses prospective acquisitions. Shepherd has taught marketing and product development courses and is a member of the Aligo Medtech Investment Committee (www.msbiv.com). She can be reached at 855-343-3100, ext. 102, or at mshepherd@medi-vantage.com. Visit her website at www.medi-vantage.com.
Additionally, in 2003, fee-for-service was the standard of care and almost no one knew about Accountable Care Organizations. Price premiums for incremental changes in medical devices were still the norm, and most hospitals had never heard of a Value Analysis Committee. The rise of digital health technology was a gleam in the eye of an entrepreneur, and most of us still used our Blackberries for placing a call and an occasional check of email.
Why This Is Important
Despite all the progress we have made, medical device technology is still in the early innovation stage. The next 15 years are going to bring even more change to the practice of providing healthcare and our role in improving healthcare with new medical technology. The changes in innovation are expected to drive growth in the global medical device market from 2017’s $403 billion to $522 billion in 2022,2 representing a healthy CAGR of 6.7 percent. Consistent global figures for the worldwide medical device market in 2003 are difficult to find, but back then, the standard measure was to multiply the U.S. market for medical devices by two to estimate the size of the global market. Using this calculation yields a 2003 global market for medical devices of $204.2 billion3 (Table 1).

Table 1: Worldwide growth in the medical device market2,3
Price Strategies Have Become Critical in Medtech
Fifteen years ago, an incremental feature improvement in a medtech product could result in a 5 percent price increase with no questions asked by healthcare. Today, those days are long since over. Further, bringing new medical devices to market is much more expensive. In an analysis of the estimated costs of bringing a PMA product to market, a 2010 Stanford study4 showed the cost of regulatory approval is high and also cited the cost of a PMA in the 1990s to be between $30 to $40 million (Table 2). This means price strategy, from simple qualitative research to a conjoint analysis, while expensive, can be a critical component of a new product strategy to ensure medical device companies earn every penny a new innovative technology deserves.

Table 2: Changes in the cost of a PMA4
Mobile Health Apps Didn’t Exist in 2003
The global mHealth market was forecasted to reach $21.5 billion by 20185 (Table 3). mHealth apps have improved in the last five years with the increasing population of tablet and smartphone users, even in the elderly segment (>65 years old)—great news for our digital health clients! Other products widely used are wearable devices, such as Fitbits, glucose sensors, and digital blood pressure monitors.

Table 3: Global mHealth growth5
Expect to see some of these apps on your phone to monitor changes in physiology. Further, look for broader adoption by patients and healthcare providers. Patients get the benefit of easier communication with their healthcare provider and more information on symptoms and medical conditions, which decreases anxiety and reduces the communications burden on healthcare providers. Healthcare providers can expect to see lower patient utilization of services (such as unnecessary emergency room visits) and a focus on improving outcomes and preventive medicine.
The Medi-Vantage Perspective
In almost every strategy research project we manage, we start with a review of adjacent technologies in the consumer markets. This review helps inform our strategy research, which often involves understanding how to integrate new technology, such as artificial intelligence (AI), into client product strategies. For example, at the World Medical Innovation Forum, held in April 2018, the majority of the Disruptive Dozen AI-based technologies6 that will have the greatest impact on healthcare in the next decade can all point to consumer-based technological advances that provided these platforms. There is no need to re-invent the wheel; there are plenty of good technological advances in development that can be modified to assist in medtech. Consider that in your product development strategy over the next 15 years.
References
- The Market for Neurotechnology: 2018-2022 published by Neurotech Reports. www.neurotechreports.com
- http://bit.ly/mpo180601
- http://bit.ly/mpo180602
- http://bit.ly/mpo180603 [PDF]
- http://bit.ly/mpo180604 [PDF]
- http://bit.ly/mpo180605
Maria Shepherd has more than 20 years of leadership experience in medical device/life-science marketing in small startups and top-tier companies. After her industry career, including her role as VP of marketing for Oridion Medical where she boosted the company valuation prior to its acquisition by Covidien/Medtronic, director of marketing for Philips Medical, and senior management roles at Boston Scientific Corp., she founded Medi-Vantage. Medi-Vantage provides marketing and business strategy as well as innovation research for the medical device industry. The firm quantitatively and qualitatively sizes and segments opportunities, evaluates new technologies, provides marketing services, and assesses prospective acquisitions. Shepherd has taught marketing and product development courses and is a member of the Aligo Medtech Investment Committee (www.msbiv.com). She can be reached at 855-343-3100, ext. 102, or at mshepherd@medi-vantage.com. Visit her website at www.medi-vantage.com.



























