Sam Brusco, Associate Editor04.03.18
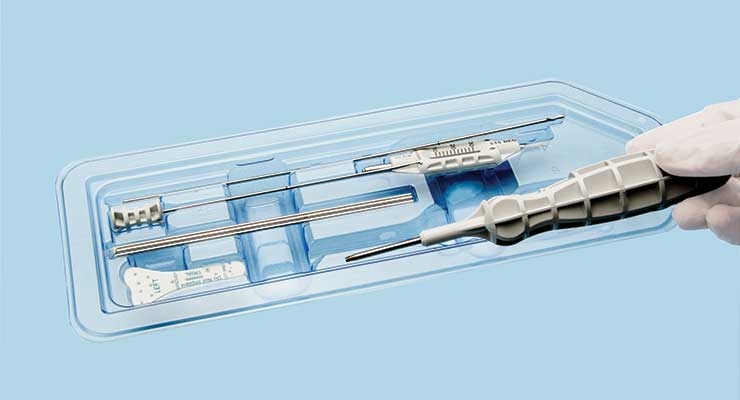
Each year, duodenoscopes are used in over 500,000 procedures as a less invasive means than traditional surgery to drain fluids from pancreatic and biliary ducts that are obstructed by cancerous tumors, gallstones, or other gastrointestinal ailments. The modern, flexible, lighted duodenoscope—inserted through the mouth into the entrance of the small intestine—is a complex medical device containing numerous small working components that present a challenge during reprocessing. If improperly sterilized, the duodenoscope may retain contaminated tissue or fluid in the crevices between all the working parts.
That risk manifested in the severest manner in two Los Angeles, Calif., hospitals (Ronald Reagan UCLA and Cedars-Sinai), when an outbreak of carbapanem-resistant Enterobacteriae (CRE)—also known as a “superbug”—struck 11 patients, killing two. The culprit was, ironically, the fault of incomplete sterilization of an elevator channel on an Olympus scope that was recently modified to render it more resistant to infection.
One remedy to hospital-acquired infections (HAIs) like CRE creeping into these devices emerged in last December’s release of Pentax Medical’s ED34-i0T model duodenoscope. ED34-i0T features a single-use, detachable distal cap—a component that rests on the tip of the device’s working end, housing the microcamera. It’s also in the most at-risk position to scoop-up infected tissue or fluid that could hide from reprocessing efforts.
“We believe the new disposable distal cap represents a major step towards lowering the risk of future infections associated with these devices,” William Maisel, M.D., M.P.H., acting director of the Office of Device Evaluation in the FDA’s Center for Devices and Radiological Health, said in an FDA statement. “Improving the safety of duodenoscopes is a top priority for the FDA, and we encourage companies to continue to pursue innovations that will help reduce the risk to patients.”
The issue isn’t exclusive to endoscopes—instruments and components spanning the cardiovascular, neurological, orthopedic, and general surgery disciplines could pose a threat for infection if complete sterilization isn’t achieved. Many different types of reusable medical devices can be costly and are subject to the potentially labor-intensive and complicated process of reprocessing. Disposable instruments or parts are one means to make the process more convenient, because those products or kits are typically pre-sterilized and individually packaged.
“Disposable medical devices vary widely across the medical specialties in terms of size, shape, and functional and performance requirements, but share some common design elements,” explained Jerome Jackson, vice president of research and development for Stellartech Research Corporation, a Milpitas, Calif.-based firm that specializes in the design, development, and contract manufacturing of sophisticated medical systems utilizing radiofrequency, ultrasound, and other energy sources. Typically combining a disposable patient-contact device and a microprocessor-controlled instrument, these systems incorporate complex electronics for sensor feedback and controlled delivery of therapeutic energy.
“Innovation has generally been driven by the interventional cardiology field, and has been adapted to the other medical specialties,” Jackson went on. “Disposable medical devices increase the number of patients that can be treated, as they do not require the lengthy cleaning and resterilization processes associated with reusable equipment. The miniaturization of disposable therapeutic devices that are used in cardiac interventional procedures, (e.g. coronary angioplasty and coronary stent placement, intravascular cardiac ablation procedures, minimally invasive cardiac valve replacement procedures, minimally invasive surgical procedures, etc.) has led to advancements in other clinical applications. For example, neurological microvascular repair and vessel stenting procedures are performed using catheters that are similar to interventional cardiology angioplasty and stent deployment catheters. Gastroenterological procedures to treat the precursor to esophageal cancer are performed using balloon deployable electrodes that deliver RF energy to the tissue to ablate metaplastic and dysplastic endothelial cell layers in much the same manner as RF ablation is used to cure cardiac arrhythmias. For these procedures, the catheter construction techniques and deployment balloon materials are similar to those used in catheters for interventional cardiac procedures.”
According to a Freedonia Industry Study, demand for disposable medical supplies in the United States will grow to $54.1 billion by 2020, with drug delivery and related products expected to be the largest and fastest-growing segment within single-use devices and components. The use of disposable medical devices or components has the potential to lower costs via logistics and reprocessing savings. They can also increase surgical efficiency and reduce the spread of infection since single-use instruments are pre-packaged, sterilized, and calibrated prior to the procedure.
Ambulatory surgery centers—known as ASCs—are modern healthcare facilities that focus on providing same-day surgical care, in addition to diagnostic and preventive procedures. The rise of ASCs have revolutionized the outpatient experience by supplying patients with an expedient alternative to hospital-based outpatient procedures. Thanks to advances in surgical techniques, anesthesia, and pain management, patients have the option to safely and comfortably recover from surgical procedures in their own homes.
According to the Ambulatory Surgery Center Association, ASCs perform over 25 million operations each year. Because single-use surgical instruments require no reprocessing and re-sterilization, they experience a much quicker turnaround time in the OR—i.e., the ability to perform more surgeries per day. Both ASCs and high-volume hospital outpatient centers could potentially benefit in the capacities of both time management and cost savings by including an increased mix of single-use instruments in their inventory.
“Continued growth of ASCs seems to favor single-use solutions,” commented Dane Waund, global marketing manager of healthcare for Solvay, an Alpharetta, Ga.-based multi-specialty chemical company whose products and solutions are used in planes, cars, smart and medical devices, batteries, in mineral and oil extraction, among many other applications promoting sustainability. “We predict products related to ASC growth to move toward single-use instrumentation. One particularly likely area would be spinal surgery, as those instrument kits contain less instruments as a rule. Early adopters have already demonstrated the efficacy of single-use instrumentation within the space.”
Single-use instruments are pristine, sterile, and calibrated before they even enter the surgeon’s hands. This reduces time spent in the OR by ensuring every procedure is being performed with an optimized tool, and that every implant has the means to be flawlessly secured. Single-use instruments also allow more efficient time management in other ways across the spectrum of those making, handling, or being operated on with the instrument.
“Our estimates suggest a savings of over $1,000 per procedure for implant firms as they reduce costs of sales per transaction,” noted James Schultz, executive vice president of sales and marketing for ECA Medical Instruments, a Thousand Oaks, Calif.-based designer and manufacturer of single-use torque-limiting surgical instruments, fixed drivers, and customized implant fixation kits. “This includes logistics and sales rep labor cost, spare and repair, and related lifecycle costs.”
Schultz also echoed the benefit to ASCs that could result from increased adoption of single-use instruments, citing both internal research from his company as well as published, peer-reviewed studies.
“Single-use instruments and products are also big savers for hospitals and ambulatory surgical centers,” he continued. “Our estimates suggest an ASC or rural hospital can save over $1,200 per procedure with an optimized instrument set that can reduce OR time—time spent in the OR cost over $37 per minute according to a recent JAMA study. OR team efficiency can also be improved, reducing the time waiting for instruments.”
Rather than a lack of time or manpower to reprocess medical products, some healthcare facilities—mostly those in remote areas—may not have the equipment necessary to adequately sterilize and reprocess their instruments.
“Disposable products improve access to medical care, especially in remote areas,” stated Jackson. “Reusing medical devices requires sophisticated cleaning and sterilizing equipment. Remote areas may not have this type of equipment and patient health can be compromised if the products are not properly cleaned and sterilized.”
The manner of production is also a consideration—hospitals or other healthcare facilities purchasing single-use instruments are going to do so in bulk, and will have to re-order approximately the same amount in future purchases. As a result, there is a great deal of opportunity for employment on the production floor for manufacturers specializing in single-use components or devices in order to ensure the constant flow of production does not stall.
“Additionally, there is a benefit to society from the jobs this industry creates,” Jackson added. “These products require ongoing component production and assembly operations, as opposed to reusable devices. This is a source of many jobs in the manufacturing sector.”
Disposable medical device sensors are also gaining traction as cost-effective tools for diagnosis, monitoring vital parameters, and a number of therapeutic applications. According to B2B research firm Markets and Markets, the market for disposable sensors is poised to reach $5.9 million by 2020. This is related to the rising incidence of HAIs, ballooning healthcare costs, venture capital firms’ increasing investments in the development of disposable, sensor-based devices, and government support for research and development activities for these devices.
Over the years, sensor-based devices have evolved with regard to characteristics, performance, and features as a result of continued improvement in sophistication of electronics in medical applications. The IT revolution, wireless sensor technology, disposable sensors, and biosensors in healthcare has provoked increasing integration of low-cost sensors designed to be disposed into medical devices—both in on-site healthcare environments and remote patient monitoring scenarios. In addition to the aforementioned improvements regarding hygiene, single-use sensors can help to limit resource constraints, improve data integrity, and reduce the cost of a number of processes.
Single-use sensors come in many forms and application areas. They can be wearable, implantable, or ingestible. In the patient monitoring field, they are employed for continuous blood pressure monitoring, implantable loop recorders, cardiac monitoring electrodes, pulse oximeters, ingestible “smart pills,” or continuous glucose blood monitoring. For diagnostics, they can be used in capsule endoscopy and all manner of diagnostic test strips. Single-use sensors can be found in some insulin pumps, cardiac therapeutic electrodes, and cardiac catheters for therapeutic applications. And any of these are found in hospitals, home-care environments, diagnostic laboratories, and clinics.
“A single-use liquid flow sensor like the LD20 comes in contact with body fluids during drug delivery therapies or when continuously measuring a patient’s urine outflow for acute kidney injury detection,” explained Susanne Jungmann, product manager for Sensirion AG, a Stäfa, Switzerland-based manufacturer of digital microsensors and systems. “There is no risk of cross-contamination with a single-use liquid or gas flow sensor, but there is for a potential reusable sensor in the case of unsuccessful reprocessing. Such processes are time consuming, may complicate logistics and processes for the hospital, and can be expensive in terms of water and energy consumption. A single-use liquid or gas flow sensor allows—and sometimes even enables—applications for the first time where reprocessing would be very difficult.”
Factors like point-of-care solutions, patient compliance, complex administering of drugs, and wearable designs demand technologies that aim to make therapies more effective can also benefit from integration of a single-use sensor. For example, a single-use differential pressure sensor designed for measuring differential pressure and mass flow has applications in medical home care, portable devices, and smart inhalers. Sensors like Sensirion’s can also be integrated into “smart” devices thanks to something called CMOSens technology. This allows integration of the sensor and signal processing circuitry on a tiny CMOS silicon chip.
“A single-use gas flow sensor also enables many new applications, a prominent example being smart inhalers that monitor and support correct inhaler use for patients. Today, six out of 10 asthma patients misuse their inhaler,” added Jungmann. “Thus, they only partially benefit from the prescribed treatment, which can improve their quality of life and the outcome of their disease. With the help of a gas flow sensor, such as the SDP3x, the patient’s inhalation can be monitored and drug release can be triggered automatically at the optimal point in time. This helps improve the patient’s compliance to the prescribed treatment, the effectiveness of the therapy, and as a result, the patient’s outcome and quality of life.”
In addition to improving the welfare of the patient, there is economic benefit behind using disposable liquid flow sensors. Preventable adverse drug events resulting from infusion pump incidents can cost hospitals thousands of dollars, and using single-use flow sensors leads to simpler designs, increasing safety and reliability. Failure modes like free flow (unhindered drug passage to the patient, causing potential overdose), open line (repeated mechanical stress or improper handling of the infusion set, causing tearing in the tubing or disconnection of fluidic adapters), or clogging (speaks for itself) can be accurately detected as well, reducing false alarms and improving the awareness of actual failures.
However, one issue that must be addressed concerning the increased usage of single-use devices and components is the consequent hike in the amount of biomedical waste, an inevitable byproduct of the healthcare industry. Once used, disposable instruments and components fall into the category of biomedical waste and must be discarded accordingly. Improperly managing this waste could result in leaks leading to contamination and infection, raising the argument that single-use medical products are not ultimately environmentally friendly.
Firms that manufacture single-use technologies are undoubtedly aware of this, and have offered solutions to remediate the issue.
“An area for potential reduction in waste may be in packaging or kitting devices used together in one tray or package instead of packaging each of the devices individually,” suggested Kristin Benokraitis, director of quality and operations for EG-GILERO, a Morrisville, N.C.-based firm that specializes in the design, development, and manufacturing within the medical devices and drug delivery system markets. “Another area may be to design the device to have a small disposable element that interfaces with a reusable device. In this case, the disposable component would minimize biological waste, and the reusable portion could be reprocessed.”
Most single-use devices and components are composed of materials that can be recycled. Thanks to the increased focus on patient-centered care and accountability, recycling is becoming the preferred management method for single-use medical products. Devices or components that were normally destined to end up in a landfill or for treatment as medical waste could be collected in the operating room or sterile processing department, then shipped off for recycling. In fact, many institutions that use a higher volume of single-use devices already have processes in place to account for the increased waste. While at first thought, this strategy would seem to inflict a heavier cost burden on those practicing it, the efforts can minimize waste disposal costs and provoke greater savings.
“Many single-use devices are manufactured using biodegradable or recyclable materials,” said Schultz. “Hospitals and ASCs have partnerships with recycling and reprocessing firms to manage medical waste. Reprocessing firms are classified as FDA-registered medical device manufacturers, and are held to the same standards for cleanliness, sterilization, and functionality as the OEM. After a device is used at a facility, it goes through extensive steps including collection, cleaning, refurbishing, quality review and testing, packaging and labeling, then sterilization to become a reprocessed device. Recycling of medical products is also growing. Specialized companies, by some estimates, are reducing operating room waste disposal costs by up to 70 percent.”
Manufacturers must also pay close attention to the materials composing their devices and components to minimize the amount of biomedical waste generated from single-use devices and components. The Restriction of Hazardous Substances Directive EU legislation—RoHS for short—was implemented in 2006 to restrict use of six hazardous materials in the manufacture of various types of electronics and electrical equipment. Medical devices were exempt in the original directive. However, 2011’s RoHS 2 directive narrowed the exemption’s scope to active implantable medical devices. RoHS 3 launched in 2015, restricts four new phthalates for in-vitro diagnostic and medical devices.
“There are a number of ways of managing the environmental impact of disposable devices,” said Jackson. “Materials that comply with the RoHS directive can be utilized to minimize contamination from heavy metals and toxic materials. Packaging can also be designed to minimize the amount and bulkiness of material that does not rapidly break down. For example, Tyvek pouches can be used as opposed to thermoformed trays, coated cardboard could replace plastic packaging trays and mounting cards, or the sterile patient-contact portion of the device could be designed to minimize size and adapt to a reusable element on an instrument rather than making the entire device disposable.”
One interesting point to make in the environmental debate is that reprocessing a medical product can actually be more impactful than disposing of it after use. This is where some of the “hidden costs” of reusable instruments begin to emerge in a significant way. Excluding environmental cost for the moment, there are the monetary costs of transfer both off site to the location for reprocessing and then back to the healthcare facility. There are also the manpower costs to reprocess the instrument, the cost of staff to arrange for reprocessing to take place, and the collection and reassembling of instruments.
But of greatest significance to the environment is the cost of the resources required to reprocess a medical product. Each sterilization method comes with its own caveats: energy is required to make steam, the use of potentially hazardous chemicals is necessary for cleaning, and the operation of machinery required for sterilization uses a large amount of power.
“We’ve done some work analyzing this with our customers, using ISO standardized analysis techniques comparing the carbon footprint of reusable and single-use instrument systems,” explained Waund. “Cradle to grave, the outcomes we have found to date are scenario specific, but in many cases, a conversion from reusable to single-use can be justified as environmentally responsible and sound, based on relevant, fact-based data.”
Even if in some cases the environmental footprint of reprocessing reusable devices exceeds that of adopting a single-use solution, there’s still a burden on those making the devices (or those making components to those devices, or the materials composing them) to devise a strategy to reduce medical waste.
“Specific to waste, we are investigating options to create circular economic solutions for our specialty polymers used in healthcare,” Waund continued. “Our corporation is committed to moving from ‘take, make, and dispose’ toward more responsible alternatives. We are very interested in collaborating with our customers and partners on concrete solutions to reduce biomedical waste.”
Reprocessed electronics may also have a greater impact on the environment than their disposable counterparts. Electronics integrated into a device can be a challenge to sterilize, requiring the use of costly resources to clean. Further, a sensor designed to be disposed of will only incorporate the most essential electronics, reducing the amount of total waste.
“Sometimes disposables or single-use components are the preferred or only technically suitable solution,” declared Jungmann. “At first glance, it might seem that single-use solutions always have a non-beneficial side regarding their environmental impact. This is too short-sighted, as this comparison does not take the entire environmental impact of reusable sensors into account. Reusable sensors use energy and resources for reprocessing, and often, due to their choice of materials, use more resources during production and end-of-life disposal. An important point can also be found in single-use components’ product design requirements. Very compact and cost-effective designs are required to meet the market volume and cost demand, which inherently works in advantage of the environment. Specifically, to make a successful single-use sensor, only electronics that are absolutely essential for the functioning and performance of the device are integrated.”
That risk manifested in the severest manner in two Los Angeles, Calif., hospitals (Ronald Reagan UCLA and Cedars-Sinai), when an outbreak of carbapanem-resistant Enterobacteriae (CRE)—also known as a “superbug”—struck 11 patients, killing two. The culprit was, ironically, the fault of incomplete sterilization of an elevator channel on an Olympus scope that was recently modified to render it more resistant to infection.
One remedy to hospital-acquired infections (HAIs) like CRE creeping into these devices emerged in last December’s release of Pentax Medical’s ED34-i0T model duodenoscope. ED34-i0T features a single-use, detachable distal cap—a component that rests on the tip of the device’s working end, housing the microcamera. It’s also in the most at-risk position to scoop-up infected tissue or fluid that could hide from reprocessing efforts.
“We believe the new disposable distal cap represents a major step towards lowering the risk of future infections associated with these devices,” William Maisel, M.D., M.P.H., acting director of the Office of Device Evaluation in the FDA’s Center for Devices and Radiological Health, said in an FDA statement. “Improving the safety of duodenoscopes is a top priority for the FDA, and we encourage companies to continue to pursue innovations that will help reduce the risk to patients.”
The issue isn’t exclusive to endoscopes—instruments and components spanning the cardiovascular, neurological, orthopedic, and general surgery disciplines could pose a threat for infection if complete sterilization isn’t achieved. Many different types of reusable medical devices can be costly and are subject to the potentially labor-intensive and complicated process of reprocessing. Disposable instruments or parts are one means to make the process more convenient, because those products or kits are typically pre-sterilized and individually packaged.
“Disposable medical devices vary widely across the medical specialties in terms of size, shape, and functional and performance requirements, but share some common design elements,” explained Jerome Jackson, vice president of research and development for Stellartech Research Corporation, a Milpitas, Calif.-based firm that specializes in the design, development, and contract manufacturing of sophisticated medical systems utilizing radiofrequency, ultrasound, and other energy sources. Typically combining a disposable patient-contact device and a microprocessor-controlled instrument, these systems incorporate complex electronics for sensor feedback and controlled delivery of therapeutic energy.
“Innovation has generally been driven by the interventional cardiology field, and has been adapted to the other medical specialties,” Jackson went on. “Disposable medical devices increase the number of patients that can be treated, as they do not require the lengthy cleaning and resterilization processes associated with reusable equipment. The miniaturization of disposable therapeutic devices that are used in cardiac interventional procedures, (e.g. coronary angioplasty and coronary stent placement, intravascular cardiac ablation procedures, minimally invasive cardiac valve replacement procedures, minimally invasive surgical procedures, etc.) has led to advancements in other clinical applications. For example, neurological microvascular repair and vessel stenting procedures are performed using catheters that are similar to interventional cardiology angioplasty and stent deployment catheters. Gastroenterological procedures to treat the precursor to esophageal cancer are performed using balloon deployable electrodes that deliver RF energy to the tissue to ablate metaplastic and dysplastic endothelial cell layers in much the same manner as RF ablation is used to cure cardiac arrhythmias. For these procedures, the catheter construction techniques and deployment balloon materials are similar to those used in catheters for interventional cardiac procedures.”
According to a Freedonia Industry Study, demand for disposable medical supplies in the United States will grow to $54.1 billion by 2020, with drug delivery and related products expected to be the largest and fastest-growing segment within single-use devices and components. The use of disposable medical devices or components has the potential to lower costs via logistics and reprocessing savings. They can also increase surgical efficiency and reduce the spread of infection since single-use instruments are pre-packaged, sterilized, and calibrated prior to the procedure.
Ambulatory surgery centers—known as ASCs—are modern healthcare facilities that focus on providing same-day surgical care, in addition to diagnostic and preventive procedures. The rise of ASCs have revolutionized the outpatient experience by supplying patients with an expedient alternative to hospital-based outpatient procedures. Thanks to advances in surgical techniques, anesthesia, and pain management, patients have the option to safely and comfortably recover from surgical procedures in their own homes.
According to the Ambulatory Surgery Center Association, ASCs perform over 25 million operations each year. Because single-use surgical instruments require no reprocessing and re-sterilization, they experience a much quicker turnaround time in the OR—i.e., the ability to perform more surgeries per day. Both ASCs and high-volume hospital outpatient centers could potentially benefit in the capacities of both time management and cost savings by including an increased mix of single-use instruments in their inventory.
“Continued growth of ASCs seems to favor single-use solutions,” commented Dane Waund, global marketing manager of healthcare for Solvay, an Alpharetta, Ga.-based multi-specialty chemical company whose products and solutions are used in planes, cars, smart and medical devices, batteries, in mineral and oil extraction, among many other applications promoting sustainability. “We predict products related to ASC growth to move toward single-use instrumentation. One particularly likely area would be spinal surgery, as those instrument kits contain less instruments as a rule. Early adopters have already demonstrated the efficacy of single-use instrumentation within the space.”
Single-use instruments are pristine, sterile, and calibrated before they even enter the surgeon’s hands. This reduces time spent in the OR by ensuring every procedure is being performed with an optimized tool, and that every implant has the means to be flawlessly secured. Single-use instruments also allow more efficient time management in other ways across the spectrum of those making, handling, or being operated on with the instrument.
“Our estimates suggest a savings of over $1,000 per procedure for implant firms as they reduce costs of sales per transaction,” noted James Schultz, executive vice president of sales and marketing for ECA Medical Instruments, a Thousand Oaks, Calif.-based designer and manufacturer of single-use torque-limiting surgical instruments, fixed drivers, and customized implant fixation kits. “This includes logistics and sales rep labor cost, spare and repair, and related lifecycle costs.”
Schultz also echoed the benefit to ASCs that could result from increased adoption of single-use instruments, citing both internal research from his company as well as published, peer-reviewed studies.
“Single-use instruments and products are also big savers for hospitals and ambulatory surgical centers,” he continued. “Our estimates suggest an ASC or rural hospital can save over $1,200 per procedure with an optimized instrument set that can reduce OR time—time spent in the OR cost over $37 per minute according to a recent JAMA study. OR team efficiency can also be improved, reducing the time waiting for instruments.”
Rather than a lack of time or manpower to reprocess medical products, some healthcare facilities—mostly those in remote areas—may not have the equipment necessary to adequately sterilize and reprocess their instruments.
“Disposable products improve access to medical care, especially in remote areas,” stated Jackson. “Reusing medical devices requires sophisticated cleaning and sterilizing equipment. Remote areas may not have this type of equipment and patient health can be compromised if the products are not properly cleaned and sterilized.”
The manner of production is also a consideration—hospitals or other healthcare facilities purchasing single-use instruments are going to do so in bulk, and will have to re-order approximately the same amount in future purchases. As a result, there is a great deal of opportunity for employment on the production floor for manufacturers specializing in single-use components or devices in order to ensure the constant flow of production does not stall.
“Additionally, there is a benefit to society from the jobs this industry creates,” Jackson added. “These products require ongoing component production and assembly operations, as opposed to reusable devices. This is a source of many jobs in the manufacturing sector.”
Disposable medical device sensors are also gaining traction as cost-effective tools for diagnosis, monitoring vital parameters, and a number of therapeutic applications. According to B2B research firm Markets and Markets, the market for disposable sensors is poised to reach $5.9 million by 2020. This is related to the rising incidence of HAIs, ballooning healthcare costs, venture capital firms’ increasing investments in the development of disposable, sensor-based devices, and government support for research and development activities for these devices.
Over the years, sensor-based devices have evolved with regard to characteristics, performance, and features as a result of continued improvement in sophistication of electronics in medical applications. The IT revolution, wireless sensor technology, disposable sensors, and biosensors in healthcare has provoked increasing integration of low-cost sensors designed to be disposed into medical devices—both in on-site healthcare environments and remote patient monitoring scenarios. In addition to the aforementioned improvements regarding hygiene, single-use sensors can help to limit resource constraints, improve data integrity, and reduce the cost of a number of processes.
Single-use sensors come in many forms and application areas. They can be wearable, implantable, or ingestible. In the patient monitoring field, they are employed for continuous blood pressure monitoring, implantable loop recorders, cardiac monitoring electrodes, pulse oximeters, ingestible “smart pills,” or continuous glucose blood monitoring. For diagnostics, they can be used in capsule endoscopy and all manner of diagnostic test strips. Single-use sensors can be found in some insulin pumps, cardiac therapeutic electrodes, and cardiac catheters for therapeutic applications. And any of these are found in hospitals, home-care environments, diagnostic laboratories, and clinics.
“A single-use liquid flow sensor like the LD20 comes in contact with body fluids during drug delivery therapies or when continuously measuring a patient’s urine outflow for acute kidney injury detection,” explained Susanne Jungmann, product manager for Sensirion AG, a Stäfa, Switzerland-based manufacturer of digital microsensors and systems. “There is no risk of cross-contamination with a single-use liquid or gas flow sensor, but there is for a potential reusable sensor in the case of unsuccessful reprocessing. Such processes are time consuming, may complicate logistics and processes for the hospital, and can be expensive in terms of water and energy consumption. A single-use liquid or gas flow sensor allows—and sometimes even enables—applications for the first time where reprocessing would be very difficult.”
Factors like point-of-care solutions, patient compliance, complex administering of drugs, and wearable designs demand technologies that aim to make therapies more effective can also benefit from integration of a single-use sensor. For example, a single-use differential pressure sensor designed for measuring differential pressure and mass flow has applications in medical home care, portable devices, and smart inhalers. Sensors like Sensirion’s can also be integrated into “smart” devices thanks to something called CMOSens technology. This allows integration of the sensor and signal processing circuitry on a tiny CMOS silicon chip.
“A single-use gas flow sensor also enables many new applications, a prominent example being smart inhalers that monitor and support correct inhaler use for patients. Today, six out of 10 asthma patients misuse their inhaler,” added Jungmann. “Thus, they only partially benefit from the prescribed treatment, which can improve their quality of life and the outcome of their disease. With the help of a gas flow sensor, such as the SDP3x, the patient’s inhalation can be monitored and drug release can be triggered automatically at the optimal point in time. This helps improve the patient’s compliance to the prescribed treatment, the effectiveness of the therapy, and as a result, the patient’s outcome and quality of life.”
In addition to improving the welfare of the patient, there is economic benefit behind using disposable liquid flow sensors. Preventable adverse drug events resulting from infusion pump incidents can cost hospitals thousands of dollars, and using single-use flow sensors leads to simpler designs, increasing safety and reliability. Failure modes like free flow (unhindered drug passage to the patient, causing potential overdose), open line (repeated mechanical stress or improper handling of the infusion set, causing tearing in the tubing or disconnection of fluidic adapters), or clogging (speaks for itself) can be accurately detected as well, reducing false alarms and improving the awareness of actual failures.
However, one issue that must be addressed concerning the increased usage of single-use devices and components is the consequent hike in the amount of biomedical waste, an inevitable byproduct of the healthcare industry. Once used, disposable instruments and components fall into the category of biomedical waste and must be discarded accordingly. Improperly managing this waste could result in leaks leading to contamination and infection, raising the argument that single-use medical products are not ultimately environmentally friendly.
Firms that manufacture single-use technologies are undoubtedly aware of this, and have offered solutions to remediate the issue.
“An area for potential reduction in waste may be in packaging or kitting devices used together in one tray or package instead of packaging each of the devices individually,” suggested Kristin Benokraitis, director of quality and operations for EG-GILERO, a Morrisville, N.C.-based firm that specializes in the design, development, and manufacturing within the medical devices and drug delivery system markets. “Another area may be to design the device to have a small disposable element that interfaces with a reusable device. In this case, the disposable component would minimize biological waste, and the reusable portion could be reprocessed.”
Most single-use devices and components are composed of materials that can be recycled. Thanks to the increased focus on patient-centered care and accountability, recycling is becoming the preferred management method for single-use medical products. Devices or components that were normally destined to end up in a landfill or for treatment as medical waste could be collected in the operating room or sterile processing department, then shipped off for recycling. In fact, many institutions that use a higher volume of single-use devices already have processes in place to account for the increased waste. While at first thought, this strategy would seem to inflict a heavier cost burden on those practicing it, the efforts can minimize waste disposal costs and provoke greater savings.
“Many single-use devices are manufactured using biodegradable or recyclable materials,” said Schultz. “Hospitals and ASCs have partnerships with recycling and reprocessing firms to manage medical waste. Reprocessing firms are classified as FDA-registered medical device manufacturers, and are held to the same standards for cleanliness, sterilization, and functionality as the OEM. After a device is used at a facility, it goes through extensive steps including collection, cleaning, refurbishing, quality review and testing, packaging and labeling, then sterilization to become a reprocessed device. Recycling of medical products is also growing. Specialized companies, by some estimates, are reducing operating room waste disposal costs by up to 70 percent.”
Manufacturers must also pay close attention to the materials composing their devices and components to minimize the amount of biomedical waste generated from single-use devices and components. The Restriction of Hazardous Substances Directive EU legislation—RoHS for short—was implemented in 2006 to restrict use of six hazardous materials in the manufacture of various types of electronics and electrical equipment. Medical devices were exempt in the original directive. However, 2011’s RoHS 2 directive narrowed the exemption’s scope to active implantable medical devices. RoHS 3 launched in 2015, restricts four new phthalates for in-vitro diagnostic and medical devices.
“There are a number of ways of managing the environmental impact of disposable devices,” said Jackson. “Materials that comply with the RoHS directive can be utilized to minimize contamination from heavy metals and toxic materials. Packaging can also be designed to minimize the amount and bulkiness of material that does not rapidly break down. For example, Tyvek pouches can be used as opposed to thermoformed trays, coated cardboard could replace plastic packaging trays and mounting cards, or the sterile patient-contact portion of the device could be designed to minimize size and adapt to a reusable element on an instrument rather than making the entire device disposable.”
One interesting point to make in the environmental debate is that reprocessing a medical product can actually be more impactful than disposing of it after use. This is where some of the “hidden costs” of reusable instruments begin to emerge in a significant way. Excluding environmental cost for the moment, there are the monetary costs of transfer both off site to the location for reprocessing and then back to the healthcare facility. There are also the manpower costs to reprocess the instrument, the cost of staff to arrange for reprocessing to take place, and the collection and reassembling of instruments.
But of greatest significance to the environment is the cost of the resources required to reprocess a medical product. Each sterilization method comes with its own caveats: energy is required to make steam, the use of potentially hazardous chemicals is necessary for cleaning, and the operation of machinery required for sterilization uses a large amount of power.
“We’ve done some work analyzing this with our customers, using ISO standardized analysis techniques comparing the carbon footprint of reusable and single-use instrument systems,” explained Waund. “Cradle to grave, the outcomes we have found to date are scenario specific, but in many cases, a conversion from reusable to single-use can be justified as environmentally responsible and sound, based on relevant, fact-based data.”
Even if in some cases the environmental footprint of reprocessing reusable devices exceeds that of adopting a single-use solution, there’s still a burden on those making the devices (or those making components to those devices, or the materials composing them) to devise a strategy to reduce medical waste.
“Specific to waste, we are investigating options to create circular economic solutions for our specialty polymers used in healthcare,” Waund continued. “Our corporation is committed to moving from ‘take, make, and dispose’ toward more responsible alternatives. We are very interested in collaborating with our customers and partners on concrete solutions to reduce biomedical waste.”
Reprocessed electronics may also have a greater impact on the environment than their disposable counterparts. Electronics integrated into a device can be a challenge to sterilize, requiring the use of costly resources to clean. Further, a sensor designed to be disposed of will only incorporate the most essential electronics, reducing the amount of total waste.
“Sometimes disposables or single-use components are the preferred or only technically suitable solution,” declared Jungmann. “At first glance, it might seem that single-use solutions always have a non-beneficial side regarding their environmental impact. This is too short-sighted, as this comparison does not take the entire environmental impact of reusable sensors into account. Reusable sensors use energy and resources for reprocessing, and often, due to their choice of materials, use more resources during production and end-of-life disposal. An important point can also be found in single-use components’ product design requirements. Very compact and cost-effective designs are required to meet the market volume and cost demand, which inherently works in advantage of the environment. Specifically, to make a successful single-use sensor, only electronics that are absolutely essential for the functioning and performance of the device are integrated.”