Brian Gorski, Vice President, P&M Corporate Finance04.03.17
Achieving growth through acquisition is a tried-and-true strategy that continues to be a popular expansion mechanism for medical manufacturing companies. Last year was a banner one for medtech M&A (as expertly detailed by medical device industry executive and advisor Bill Ellerkamp in Medical Product Outsourcing’s January/February issue), with industry stalwarts such as Genstar Capital LLC-backed Tecomet Inc. (acquiring Mountainside Medical Colorado LLC), Linden Capital Partners backed-Flexan LLC (acquiring MEDRON Inc.), and Nordson Corporation (purchasing LinkTech Quick Couplings Inc.) executing strategic buys. Private equity platform activity continued to shape the evolving competitive landscape, with notable transactions including the JLL Partners/Water Street Healthcare Partners purchase of Medplast Inc. (from Baird Capital Partners) and Vance Street Capital LLC’s acquisitions of Motion Dynamics Corporation and A&E Medical Corporation. In short, “traditional” buyers continue to drive the majority of transaction activity.
However, recently we have seen a growing subset of “non-medical” large corporate buyers making significant forays into medical manufacturing, upping the competition for quality assets with traditional strategic and private equity buyers. The November 2016 acquisition of Tegra Medical by Swiss fastening component manufacturer SFS Group AG is the perfect example of a non-traditional buyer obtaining a highly desirable, quality medical manufacturing asset. As stated on Tegra’s website, “SFS Group wants to establish a North American presence in the medical device marketplace; by positioning us as their medical competence center, we are playing an integral role in their entrance.”
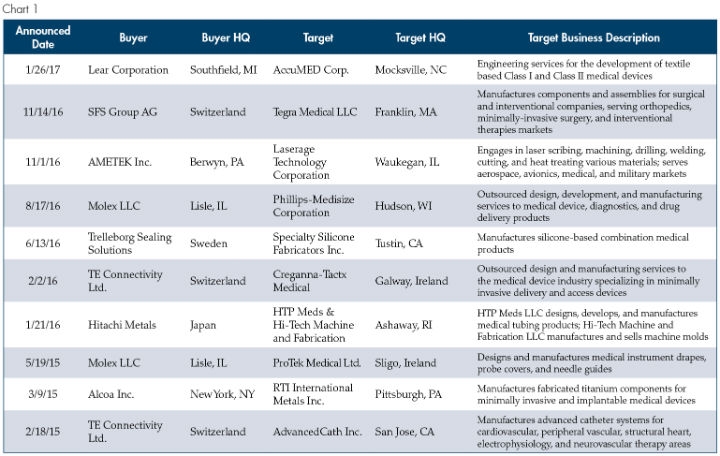
In this piece we will explore why these non-traditional buyers are drawn to medical manufacturing, the characteristics of an attractive target to this new breed of buyer, and what this dynamic means for owners and shareholders contemplating exit strategies. Before digging in, the chart below provides a snapshot of medical manufacturing transactions completed by what we would view as “non-traditional” buyers since the start of 2015.
Why Medical Manufacturing?
Industrial growth in traditional manufacturing industries is expected to face continued headwinds in the years ahead. MAPI’s December 2016 U.S. Industrial Outlook predicts less than 1 percent growth in manufacturing output in the United States through 2017, with slightly more than 1 percent through the end of 2020. Globally, the European Union’s economic malaise and China’s significantly slowed growth are challenging organic corporate growth in the United States.
Conversely, the medtech market continues to expand, driven primarily by longer life expectancy and an increased expenditure on healthcare, particularly in emerging economies. Additionally, the North American and European regulatory environments have prompted a shift to value-based healthcare solutions, which in turn, has helped increase competition, advance business models, and spark innovative strategies to achieve sustainable growth. PMCF expects the medical contract manufacturing industry to grow 10.5 percent annually through 2021.
As pressures from boards and shareholders mount to achieve revenue growth and margin targets, investing in medical device manufacturing is increasingly seen as an avenue to find top-line growth in the midst of challenging economic conditions. For example, in a January 2017 presentation for the Swiss Equities Conference, SFS Group AG cited “increased exposure to [the] attractive and growing medical device market” as the first point in explaining the rationale behind its acquisition of Tegra Medical.
Complimentary Business Models and Competencies
Medical manufacturers operate in a highly competitive and tightly regulated industry. Large companies operating in similarly exacting industries such as aerospace, electronics, or automotive, often find commonalities in philosophy and production standards as it relates to customer service. In announcing the Molex purchase of Phillips-Medisize in August 2016, Molex Senior Vice President of Business Development and Corporate Strategy Tim Ruff noted, “Phillips-Medisize has a talented, experienced and innovative team that has strong customer relationships because of its outstanding ability to serve the unique needs of the medical solutions market. Combined with Molex’s expertise in electronics and broad global manufacturing presence, we are confident that together we can significantly expand our medical solutions capabilities globally.”
Adding a specific and difficult to replicate technology or manufacturing capability is another reason large corporations are increasingly targeting medtech manufacturing. Oftentimes existing customers in adjacent industries present immediate revenue synergies with an acquisition, allowing a large corporate buyer to rapidly fill capacity and improve overhead absorption in the acquired entity. This ability to offer additional services and capabilities to existing customers deepens relationships and increases confidence and loyalty. The November 2016 union of AMETEK Inc.—a $4 billion global manufacturer of electronic instruments and electro-mechanical devices—and Laserage Technology Corporation, a laser contract manufacturing expert for medical device components with roughly $22 million in 2016 sales, is a textbook example of a large corporate buyer leveraging the specialized skillset of a niche market leader. In announcing the transaction, AMETEK CEO David A. Zapico remarked, “Laserage is an excellent addition to our growing presence in the medical industry. Its fabrication and manufacturing capabilities are an excellent fit with our engineered medical components business providing strong market and technology synergies.”
Barriers to Entry
Being selected as a product development and manufacturing partner to the world’s leading medical OEMs is no small feat, as making life saving medical instruments, implants, or devices requires a significant investment in engineering, quality systems, and cutting-edge equipment and manufacturing technology. The institutional knowledge held by seasoned engineers and highly skilled machinists can be costly to replicate in many specialized medical manufacturing facilities. Layer on expensive regulations such as ISO certifications, expensive and time-consuming U.S. Food and Drug Administration certifications, as well as customer quality audits and the rationale for buying versus building medical manufacturing capabilities becomes even more evident.
(Un)attractive Targets
In many ways, the characteristics of an attractive target for a “non-traditional” buyer are mostly the same as those valued by an industry strategic buyer or private equity firm. Top attributes include:
Sellers looking for a complete financial exit but hesitant to consider selling to a close competitor out of concern for legacy preservation, facility consolidation, or long-term advancement opportunities for key managers may find that this new set of buyers can check many of their “want” boxes. We have repeatedly seen non-traditional buyers leave newly acquired medical manufacturing assets largely (if not entirely) intact, while bringing to the table the added benefits of a large multinational corporate infrastructure (distribution channels, raw material purchasing power, etc.).
We expect the pace of consolidation and M&A activity in the medical manufacturing space to continue to accelerate. As owners and shareholders evaluate exit strategies, this new breed of non-traditional corporate buyers presents an interesting, and often attractive alternative to selling to private equity or an obvious industry buyer.
Brian Gorski is a vice president with PMCF and a member of the firm’s medical technology team. He has more than twelve years of transaction advisory experience, having advised public and private companies on merger and acquisition mandates, valuation matters, private placements and financing transactions across a variety of industries. He can be reached at brian.gorski@pmcf.com.
However, recently we have seen a growing subset of “non-medical” large corporate buyers making significant forays into medical manufacturing, upping the competition for quality assets with traditional strategic and private equity buyers. The November 2016 acquisition of Tegra Medical by Swiss fastening component manufacturer SFS Group AG is the perfect example of a non-traditional buyer obtaining a highly desirable, quality medical manufacturing asset. As stated on Tegra’s website, “SFS Group wants to establish a North American presence in the medical device marketplace; by positioning us as their medical competence center, we are playing an integral role in their entrance.”
In this piece we will explore why these non-traditional buyers are drawn to medical manufacturing, the characteristics of an attractive target to this new breed of buyer, and what this dynamic means for owners and shareholders contemplating exit strategies. Before digging in, the chart below provides a snapshot of medical manufacturing transactions completed by what we would view as “non-traditional” buyers since the start of 2015.

Why Medical Manufacturing?
Industrial growth in traditional manufacturing industries is expected to face continued headwinds in the years ahead. MAPI’s December 2016 U.S. Industrial Outlook predicts less than 1 percent growth in manufacturing output in the United States through 2017, with slightly more than 1 percent through the end of 2020. Globally, the European Union’s economic malaise and China’s significantly slowed growth are challenging organic corporate growth in the United States.
Conversely, the medtech market continues to expand, driven primarily by longer life expectancy and an increased expenditure on healthcare, particularly in emerging economies. Additionally, the North American and European regulatory environments have prompted a shift to value-based healthcare solutions, which in turn, has helped increase competition, advance business models, and spark innovative strategies to achieve sustainable growth. PMCF expects the medical contract manufacturing industry to grow 10.5 percent annually through 2021.
As pressures from boards and shareholders mount to achieve revenue growth and margin targets, investing in medical device manufacturing is increasingly seen as an avenue to find top-line growth in the midst of challenging economic conditions. For example, in a January 2017 presentation for the Swiss Equities Conference, SFS Group AG cited “increased exposure to [the] attractive and growing medical device market” as the first point in explaining the rationale behind its acquisition of Tegra Medical.
Complimentary Business Models and Competencies
Medical manufacturers operate in a highly competitive and tightly regulated industry. Large companies operating in similarly exacting industries such as aerospace, electronics, or automotive, often find commonalities in philosophy and production standards as it relates to customer service. In announcing the Molex purchase of Phillips-Medisize in August 2016, Molex Senior Vice President of Business Development and Corporate Strategy Tim Ruff noted, “Phillips-Medisize has a talented, experienced and innovative team that has strong customer relationships because of its outstanding ability to serve the unique needs of the medical solutions market. Combined with Molex’s expertise in electronics and broad global manufacturing presence, we are confident that together we can significantly expand our medical solutions capabilities globally.”
Adding a specific and difficult to replicate technology or manufacturing capability is another reason large corporations are increasingly targeting medtech manufacturing. Oftentimes existing customers in adjacent industries present immediate revenue synergies with an acquisition, allowing a large corporate buyer to rapidly fill capacity and improve overhead absorption in the acquired entity. This ability to offer additional services and capabilities to existing customers deepens relationships and increases confidence and loyalty. The November 2016 union of AMETEK Inc.—a $4 billion global manufacturer of electronic instruments and electro-mechanical devices—and Laserage Technology Corporation, a laser contract manufacturing expert for medical device components with roughly $22 million in 2016 sales, is a textbook example of a large corporate buyer leveraging the specialized skillset of a niche market leader. In announcing the transaction, AMETEK CEO David A. Zapico remarked, “Laserage is an excellent addition to our growing presence in the medical industry. Its fabrication and manufacturing capabilities are an excellent fit with our engineered medical components business providing strong market and technology synergies.”
Barriers to Entry
Being selected as a product development and manufacturing partner to the world’s leading medical OEMs is no small feat, as making life saving medical instruments, implants, or devices requires a significant investment in engineering, quality systems, and cutting-edge equipment and manufacturing technology. The institutional knowledge held by seasoned engineers and highly skilled machinists can be costly to replicate in many specialized medical manufacturing facilities. Layer on expensive regulations such as ISO certifications, expensive and time-consuming U.S. Food and Drug Administration certifications, as well as customer quality audits and the rationale for buying versus building medical manufacturing capabilities becomes even more evident.
(Un)attractive Targets
In many ways, the characteristics of an attractive target for a “non-traditional” buyer are mostly the same as those valued by an industry strategic buyer or private equity firm. Top attributes include:
- Long-term and deeply entrenched customer relationships with a diversified set of blue-chip OEMs, presenting the potential for revenue synergies with existing product and service offerings
- Proprietary or specialized manufacturing capabilities that are difficult and costly to replicate, yet applicable to complimentary customers and adjacent end markets
- Top-of-the-line and current manufacturing equipment, signifying a willingness to invest in growth
- Team of expert and long-tenured engineering, manufacturing, and sales professionals
- Best-in-class quality systems
- New entrants to medical manufacturing are less likely to be interested in companies that generate a meaningful percentage of their revenue from developmental stage projects requiring 510(k) or other regulatory approvals.
- Larger, non-traditional companies may be more willing to overlook the absence of a clear succession plan for senior leadership, often leveraging existing internal resources as an interim solution until a more permanent president or facility manager can be found. While this isn’t meaningfully divergent from how traditional strategic buyers may feel about long-term leadership, it certainly differentiates non-traditional organizations from a large percentage of financial buyers.
- Given the intricacies and cash collection dynamics of medical product distribution, non-traditional buyers are typically not interested in products or assembly processes that require last-stage delivery to a healthcare system or medical device distributor.
Sellers looking for a complete financial exit but hesitant to consider selling to a close competitor out of concern for legacy preservation, facility consolidation, or long-term advancement opportunities for key managers may find that this new set of buyers can check many of their “want” boxes. We have repeatedly seen non-traditional buyers leave newly acquired medical manufacturing assets largely (if not entirely) intact, while bringing to the table the added benefits of a large multinational corporate infrastructure (distribution channels, raw material purchasing power, etc.).
We expect the pace of consolidation and M&A activity in the medical manufacturing space to continue to accelerate. As owners and shareholders evaluate exit strategies, this new breed of non-traditional corporate buyers presents an interesting, and often attractive alternative to selling to private equity or an obvious industry buyer.
Brian Gorski is a vice president with PMCF and a member of the firm’s medical technology team. He has more than twelve years of transaction advisory experience, having advised public and private companies on merger and acquisition mandates, valuation matters, private placements and financing transactions across a variety of industries. He can be reached at brian.gorski@pmcf.com.