07.30.19
AT A GLANCE
Rank: #4 (Last year: #6)
$18.93 Billion ($30.6B total)
Prior Fiscal: $16.2 Billion
Percentage Change: +17%
No. of Employees: 103,000
Global Headquarters: Abbott Park, Ill.
KEY EXECUTIVES
Miles D. White, Chairman and CEO
Robert B. Ford, President and COO
Hubert L. Allen, Exec. VP, General Counsel and Secretary
Brian J. Blaser, Exec. VP, Diagnostics Products
John M. Capek, Ph.D., Exec. VP, Ventures
Stephen R. Fussell, Exec. VP, Human Resources
John F. Ginascol, Exec. VP, Core Diagnostics
Brian B. Yoor, Exec. VP, Finance and CFO
Chuck Brynelsen, Sr. VP, Abbott Vascular
Jaime Contreras, Sr. VP, Core Laboratory Diagnostics, Commercial Operations
Robert Funck, Sr. VP, Finance and Controller
Corlis D. Murray, Sr. VP, Quality Assurance, Regulatory and Engineering Services
Michael J. Pederson, Sr. VP, Cardiac Arrhythmias and Heart Failure
Christopher J. Scoggins, Sr. VP, Rapid Diagnostics
Jared L. Watkin, Sr. VP, Diabetes Care
Randel Woodgrift, Sr. VP, Cardiac Rhythm Management
In a January 2018 piece about the firm and its leader, Forbes senior contributor Bruce Japsen was right on the money when he stated Abbott’s CEO, Miles White, “may be setting the diversified healthcare company up for a calm year of paying down debt and investing in research and operational performance.” After experiencing back-to-back headlines-filled years in 2016 and 2017, dominated by the two multi-billion dollar acquisitions of St. Jude and Alere, as well as the subsequent ups and downs that came with both transactions—cybersecurity and “hacking” concerns with St. Jude devices, and ethical and financial issues with Alere—it’s no wonder 2018 was significantly quieter in comparison.
At the time of the Forbes article, Abbott’s debt load had recently been reduced by $4 billion from $28 billion, which represented the largest in the firm’s 130-year history (a result of the two major deals in the years prior). White expected to pay down another $4 billion before the end of 2018, noting that as a top priority. According to the firm’s 2018 annual report, the company exceeded that goal by approximately $700 million.
Without looking to conduct market-leading M&A transactions in 2018, Abbott was able to focus on organic growth through product launches and innovation. Although the firm wasn’t making much noise in business and financial headlines, that by no means meant it was operating under the radar during the year. Just ask now 4-year-old Sadie Rutenberg of Seattle, Wash., how Abbott has made an impact.
Sadie, the “cover model” for the firm’s 2018 annual report, was a participant in a clinical trial for the Masters HP 15-mm rotatable mechanical heart valve, becoming the first child in the U.S. to receive the Abbott device. The dime-sized implant—the world’s smallest mechanical heart valve for pediatric patients with heart defects—received FDA approval in March 2018 and represented just one way the organization had a substantial effect during the year.
“There’s an urgent need for the smallest babies and children who need a suitable replacement valve in order to survive,” Michael Dale, vice president of Abbott’s structural heart business, said in a news release announcing the approval. “Abbott’s new mechanical pediatric heart valve is a life-changing technology for the smallest pediatric patients, giving them a better chance at a long, healthy life with a fully functioning heart.”
In fact, the company claims in the U.S. alone, heart defects affect nearly 1 percent of births each year (about 40,000). Those involving a poorly functioning valve gained a new option with the approval.
The pediatric valve joined a family of products produced by Abbott to treat an array of heart-related conditions, housed within the firm’s Cardiovascular and Neuromodulation business. The unit, bolstered substantially by the St. Jude acquisition, contributed $9.44 billion to the organization’s 2018 $30.58 billion sales total as the largest of all of Abbott’s four major businesses.
The segment is comprised of six divisions, led in 2018 by the Vascular portion, which contributed $2.9 billion to the sales total ($1.1B from U.S. and $1.8B from international).
Following that was Rhythm Management, Electrophysiology, and Structural Heart, offering $2.1 billion ($1.0B U.S.; $1.1B intl.), $1.7 billion ($764M U.S.; $904M intl.), and $1.2 billion ($488M U.S.; $751M intl.) respectively. Only two portions were under $1 billion in sales—Neuromodulation at $864 million ($690M U.S. and $174M intl.) and Heart Failure at $646 million ($467M U.S. and $179 intl.). Perhaps not so coincidently, those two units were also the only ones of the six where U.S. sales exceeded those from abroad. Further, five of the six units saw growth over the prior fiscal year, which was $8.9 billion for the entire business in 2017, with Rhythm Management as the lone exception.
Although perhaps most newsworthy, the pediatric valve was not the only new product from Abbott’s Cardiovascular and Neuromodulation business to gain attention during its last fiscal year. Among other notable product news was:
FDA approval for magnetic resonance-conditional labeling for the Quadra Assura MP cardiac resynchronization therapy defibrillator and Fortify Assura implantable cardioverter defibrillator. The approvals follow with similar decisions by the regulatory agency regarding MRI-ready implantable cardiac solutions sold by Abbott. In addition, both the Fortify Assura and Quadra Assura MP include the company’s suite of TailoredTherapy features, which are designed to provide physicians additional flexibility and control in how they deliver therapy to treat their patients’ cardiac arrhythmias and congestive heart failure. Finally, the Quadra Assura MP also offers MultiPoint Pacing and SyncAV technology to provide additional options for patients who are not responsive to traditional cardiac resynchronization therapy.
The Advisor HD Grid Mapping Catheter with Sensor Enabled technology gained FDA clearance in May. The device’s design allows physicians to capture and analyze data in a novel manner to create highly detailed maps of the heart that better differentiate healthy from unhealthy tissue.
The DRG Invisible Trial System, which had secured both U.S. FDA approval and a European CE mark, was launched in November. The technology leverages Abbott’s dorsal root ganglion (DRG) stimulation technology, providing an opportunity for patients to try a non-opioid pain therapy before actually getting an implantable device. If the DRG therapy works for a patient, they would then have the company’s Proclaim DRG system implanted.
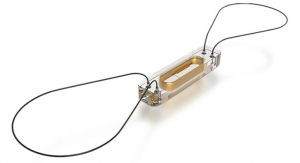
The XIENCE Sierra, the latest in the company’s line of everolimus-eluting coronary stents, received FDA approval last May. Design and technology advances in this generation of XIENCE provide features specifically designed for the treatment of complex blockages that now account for up to 70 percent of cases, according to Abbott. Those design innovations include a thinner profile, increased flexibility, longer lengths, and small diameters.
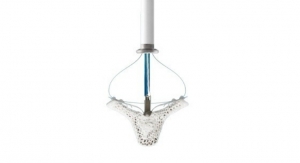
An EU CE mark was received and FDA granted approval of the firm’s next-generation version of the MitraClip heart valve repair device used to repair a leaky mitral valve without open-heart surgery. This offering provides advanced steering, navigation, and positioning capabilities, making it easier to use in difficult anatomies.
In October, the HeartMate 3 Left Ventricular Assist Device (LVAD) gained FDA approval as a destination therapy for people living with advanced heart failure, enabling the system to be used for patients not eligible for a transplant who will live with their device for the rest of their lives. While reducing the size of the LVAD, the company also employed Full MagLev (fully magnetically-levitated) Flow, which reduces trauma to the blood passing through the pump while improving flow.
Abbott’s Diagnostics business came in second in overall sales contributions at $7.5 billion, but reflected the greatest dollar increase over prior year, which was $5.6 billion in 2017. While all four units of Diagnostics saw increases in sales over the prior year, two were almost flat while a third accounted for almost the entirety of the rise. Core Laboratory enjoyed a modest increase between 2017’s $4.1 billion in sales to 2018’s $4.4 billion ($985M U.S.; $3.4B intl.). Point of Care and Molecular, virtually flat, had sales of $553M and $484M in 2018 respectively. Experiencing a huge bump in contribution, Rapid Diagnostics went from $540 million in 2017 to $2.1 billion in 2018 ($1.1B U.S.; $924M intl.). Undoubtedly, the full integration of Alere and its product line into the fold was the most significant factor in the increase year-over-year.
Similar to the Cardiovascular and Neuromodulation business, Diagnostics enjoyed positive news of its own with regard to its product offerings.
Most notable among the highlights was the CE mark awarded for the first troponin test to enable a better prediction of the chances for a heart attack or other cardiac event potentially months to years in advance in people who otherwise appear healthy. The test enables doctors to alter how they identify those at risk for developing heart disease because the diagnostic test uses a biomarker specific to the heart. With this added information, doctors can help ensure the correct treatment is given to people at high risk and prevent unnecessary testing, medication, and costs for lower-risk patients.
Across the pond, the FDA cleared Abbott’s next-generation Influenza A & B 2 and Strep A 2 molecular assays for point-of-care testing. According to the company, at the time of the clearance announcement, the Influenza A & B 2 assay offers the fastest point-of-care molecular detection and differentiation of influenza A and B virus available—13 minutes or less, with early call out of positive results in as little as five minutes. The Strep A 2 provides molecular detection of Group A Streptococcus bacterial nucleic acid more than twice as rapidly as other available molecular tests—in six minutes or less, with call out of positive results as early as two minutes.
The company also announced the first viral load point-of-care test designed to provide healthcare professionals, especially in remote and underserved communities, with a fast, accurate, and easy-to-use test to manage HIV. By providing viral load test results in less than 70 minutes, this life-changing technology allows patients to get tested and treated in the same visit.
A CE mark was received for the Alinity h-series integrated system for hematology testing. The system integrates the Alinity hq with the Alinity hs slide maker and stainer module into a combined solution. The solution is 20 percent faster per m2 than other currently available integrated hematology systems, according to Abbott, with a throughput of 133 complete blood counts (CBCs) per m2.
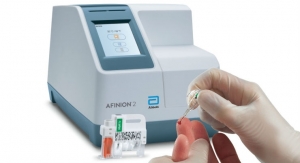
Also in 2018, the company launched its Afinion 2 analyzer in the U.S. for diabetes management. The analyzer is a compact rapid, multi-assay platform that streamlines and simplifies the delivery of actionable, accurate measurements of hemoglobin A1c (HbA1c) and albumin to creatinine ratio (ACR) results at the point of care. The system arms healthcare professionals with the information needed to make fast and accurate medical decisions—three minutes for HbA1c and five minutes for ACR—enabling healthcare professionals to dedicate more time to counseling diabetes patients within a single office visit.
Speaking of diabetes, Abbott reports on its offerings for this space as “other” in its annual report, although it’s a significant segment of the company. The products out of this portion led to just shy of $2 billion in sales, with a 25/75 percent ratio U.S./international ($493 million versus $1.5 billion). The group saw a substantial rise over 2017, which had the contribution from this segment at $1.65 billion.
Abbott’s diabetes focus is centered around its Freestyle Libre product, which was the focus of several notable headlines in 2018. Making the most waves was the announcement of its app for the iPhone that enables users to access glucose data directly from their Apple smartphone. This capability eliminated the need for users to carry a separate reader device. While the app had been available for Android users since 2015, it had just been released for the iPhone.
Also of significance was the announcement of the Freestyle Libre being made available to Medicare patients. The factory-calibrated system is the only CGM system recognized by Medicare that requires no user calibration whatsoever (either by fingerstick or manual data entry) at the time of the company’s announcement. The system also does not require the need for routine fingersticks and allows for patients to dose insulin based on the system’s results.
Rank: #4 (Last year: #6)
$18.93 Billion ($30.6B total)
Prior Fiscal: $16.2 Billion
Percentage Change: +17%
No. of Employees: 103,000
Global Headquarters: Abbott Park, Ill.
KEY EXECUTIVES
Miles D. White, Chairman and CEO
Robert B. Ford, President and COO
Hubert L. Allen, Exec. VP, General Counsel and Secretary
Brian J. Blaser, Exec. VP, Diagnostics Products
John M. Capek, Ph.D., Exec. VP, Ventures
Stephen R. Fussell, Exec. VP, Human Resources
John F. Ginascol, Exec. VP, Core Diagnostics
Brian B. Yoor, Exec. VP, Finance and CFO
Chuck Brynelsen, Sr. VP, Abbott Vascular
Jaime Contreras, Sr. VP, Core Laboratory Diagnostics, Commercial Operations
Robert Funck, Sr. VP, Finance and Controller
Corlis D. Murray, Sr. VP, Quality Assurance, Regulatory and Engineering Services
Michael J. Pederson, Sr. VP, Cardiac Arrhythmias and Heart Failure
Christopher J. Scoggins, Sr. VP, Rapid Diagnostics
Jared L. Watkin, Sr. VP, Diabetes Care
Randel Woodgrift, Sr. VP, Cardiac Rhythm Management
In a January 2018 piece about the firm and its leader, Forbes senior contributor Bruce Japsen was right on the money when he stated Abbott’s CEO, Miles White, “may be setting the diversified healthcare company up for a calm year of paying down debt and investing in research and operational performance.” After experiencing back-to-back headlines-filled years in 2016 and 2017, dominated by the two multi-billion dollar acquisitions of St. Jude and Alere, as well as the subsequent ups and downs that came with both transactions—cybersecurity and “hacking” concerns with St. Jude devices, and ethical and financial issues with Alere—it’s no wonder 2018 was significantly quieter in comparison.
At the time of the Forbes article, Abbott’s debt load had recently been reduced by $4 billion from $28 billion, which represented the largest in the firm’s 130-year history (a result of the two major deals in the years prior). White expected to pay down another $4 billion before the end of 2018, noting that as a top priority. According to the firm’s 2018 annual report, the company exceeded that goal by approximately $700 million.
Without looking to conduct market-leading M&A transactions in 2018, Abbott was able to focus on organic growth through product launches and innovation. Although the firm wasn’t making much noise in business and financial headlines, that by no means meant it was operating under the radar during the year. Just ask now 4-year-old Sadie Rutenberg of Seattle, Wash., how Abbott has made an impact.
Sadie, the “cover model” for the firm’s 2018 annual report, was a participant in a clinical trial for the Masters HP 15-mm rotatable mechanical heart valve, becoming the first child in the U.S. to receive the Abbott device. The dime-sized implant—the world’s smallest mechanical heart valve for pediatric patients with heart defects—received FDA approval in March 2018 and represented just one way the organization had a substantial effect during the year.
“There’s an urgent need for the smallest babies and children who need a suitable replacement valve in order to survive,” Michael Dale, vice president of Abbott’s structural heart business, said in a news release announcing the approval. “Abbott’s new mechanical pediatric heart valve is a life-changing technology for the smallest pediatric patients, giving them a better chance at a long, healthy life with a fully functioning heart.”
In fact, the company claims in the U.S. alone, heart defects affect nearly 1 percent of births each year (about 40,000). Those involving a poorly functioning valve gained a new option with the approval.
The pediatric valve joined a family of products produced by Abbott to treat an array of heart-related conditions, housed within the firm’s Cardiovascular and Neuromodulation business. The unit, bolstered substantially by the St. Jude acquisition, contributed $9.44 billion to the organization’s 2018 $30.58 billion sales total as the largest of all of Abbott’s four major businesses.
The segment is comprised of six divisions, led in 2018 by the Vascular portion, which contributed $2.9 billion to the sales total ($1.1B from U.S. and $1.8B from international).
Following that was Rhythm Management, Electrophysiology, and Structural Heart, offering $2.1 billion ($1.0B U.S.; $1.1B intl.), $1.7 billion ($764M U.S.; $904M intl.), and $1.2 billion ($488M U.S.; $751M intl.) respectively. Only two portions were under $1 billion in sales—Neuromodulation at $864 million ($690M U.S. and $174M intl.) and Heart Failure at $646 million ($467M U.S. and $179 intl.). Perhaps not so coincidently, those two units were also the only ones of the six where U.S. sales exceeded those from abroad. Further, five of the six units saw growth over the prior fiscal year, which was $8.9 billion for the entire business in 2017, with Rhythm Management as the lone exception.
Although perhaps most newsworthy, the pediatric valve was not the only new product from Abbott’s Cardiovascular and Neuromodulation business to gain attention during its last fiscal year. Among other notable product news was:
FDA approval for magnetic resonance-conditional labeling for the Quadra Assura MP cardiac resynchronization therapy defibrillator and Fortify Assura implantable cardioverter defibrillator. The approvals follow with similar decisions by the regulatory agency regarding MRI-ready implantable cardiac solutions sold by Abbott. In addition, both the Fortify Assura and Quadra Assura MP include the company’s suite of TailoredTherapy features, which are designed to provide physicians additional flexibility and control in how they deliver therapy to treat their patients’ cardiac arrhythmias and congestive heart failure. Finally, the Quadra Assura MP also offers MultiPoint Pacing and SyncAV technology to provide additional options for patients who are not responsive to traditional cardiac resynchronization therapy.
The Advisor HD Grid Mapping Catheter with Sensor Enabled technology gained FDA clearance in May. The device’s design allows physicians to capture and analyze data in a novel manner to create highly detailed maps of the heart that better differentiate healthy from unhealthy tissue.
The DRG Invisible Trial System, which had secured both U.S. FDA approval and a European CE mark, was launched in November. The technology leverages Abbott’s dorsal root ganglion (DRG) stimulation technology, providing an opportunity for patients to try a non-opioid pain therapy before actually getting an implantable device. If the DRG therapy works for a patient, they would then have the company’s Proclaim DRG system implanted.
The XIENCE Sierra, the latest in the company’s line of everolimus-eluting coronary stents, received FDA approval last May. Design and technology advances in this generation of XIENCE provide features specifically designed for the treatment of complex blockages that now account for up to 70 percent of cases, according to Abbott. Those design innovations include a thinner profile, increased flexibility, longer lengths, and small diameters.
An EU CE mark was received and FDA granted approval of the firm’s next-generation version of the MitraClip heart valve repair device used to repair a leaky mitral valve without open-heart surgery. This offering provides advanced steering, navigation, and positioning capabilities, making it easier to use in difficult anatomies.
In October, the HeartMate 3 Left Ventricular Assist Device (LVAD) gained FDA approval as a destination therapy for people living with advanced heart failure, enabling the system to be used for patients not eligible for a transplant who will live with their device for the rest of their lives. While reducing the size of the LVAD, the company also employed Full MagLev (fully magnetically-levitated) Flow, which reduces trauma to the blood passing through the pump while improving flow.
Abbott’s Diagnostics business came in second in overall sales contributions at $7.5 billion, but reflected the greatest dollar increase over prior year, which was $5.6 billion in 2017. While all four units of Diagnostics saw increases in sales over the prior year, two were almost flat while a third accounted for almost the entirety of the rise. Core Laboratory enjoyed a modest increase between 2017’s $4.1 billion in sales to 2018’s $4.4 billion ($985M U.S.; $3.4B intl.). Point of Care and Molecular, virtually flat, had sales of $553M and $484M in 2018 respectively. Experiencing a huge bump in contribution, Rapid Diagnostics went from $540 million in 2017 to $2.1 billion in 2018 ($1.1B U.S.; $924M intl.). Undoubtedly, the full integration of Alere and its product line into the fold was the most significant factor in the increase year-over-year.
Similar to the Cardiovascular and Neuromodulation business, Diagnostics enjoyed positive news of its own with regard to its product offerings.
Most notable among the highlights was the CE mark awarded for the first troponin test to enable a better prediction of the chances for a heart attack or other cardiac event potentially months to years in advance in people who otherwise appear healthy. The test enables doctors to alter how they identify those at risk for developing heart disease because the diagnostic test uses a biomarker specific to the heart. With this added information, doctors can help ensure the correct treatment is given to people at high risk and prevent unnecessary testing, medication, and costs for lower-risk patients.
Across the pond, the FDA cleared Abbott’s next-generation Influenza A & B 2 and Strep A 2 molecular assays for point-of-care testing. According to the company, at the time of the clearance announcement, the Influenza A & B 2 assay offers the fastest point-of-care molecular detection and differentiation of influenza A and B virus available—13 minutes or less, with early call out of positive results in as little as five minutes. The Strep A 2 provides molecular detection of Group A Streptococcus bacterial nucleic acid more than twice as rapidly as other available molecular tests—in six minutes or less, with call out of positive results as early as two minutes.
The company also announced the first viral load point-of-care test designed to provide healthcare professionals, especially in remote and underserved communities, with a fast, accurate, and easy-to-use test to manage HIV. By providing viral load test results in less than 70 minutes, this life-changing technology allows patients to get tested and treated in the same visit.
A CE mark was received for the Alinity h-series integrated system for hematology testing. The system integrates the Alinity hq with the Alinity hs slide maker and stainer module into a combined solution. The solution is 20 percent faster per m2 than other currently available integrated hematology systems, according to Abbott, with a throughput of 133 complete blood counts (CBCs) per m2.
Also in 2018, the company launched its Afinion 2 analyzer in the U.S. for diabetes management. The analyzer is a compact rapid, multi-assay platform that streamlines and simplifies the delivery of actionable, accurate measurements of hemoglobin A1c (HbA1c) and albumin to creatinine ratio (ACR) results at the point of care. The system arms healthcare professionals with the information needed to make fast and accurate medical decisions—three minutes for HbA1c and five minutes for ACR—enabling healthcare professionals to dedicate more time to counseling diabetes patients within a single office visit.
Speaking of diabetes, Abbott reports on its offerings for this space as “other” in its annual report, although it’s a significant segment of the company. The products out of this portion led to just shy of $2 billion in sales, with a 25/75 percent ratio U.S./international ($493 million versus $1.5 billion). The group saw a substantial rise over 2017, which had the contribution from this segment at $1.65 billion.
Abbott’s diabetes focus is centered around its Freestyle Libre product, which was the focus of several notable headlines in 2018. Making the most waves was the announcement of its app for the iPhone that enables users to access glucose data directly from their Apple smartphone. This capability eliminated the need for users to carry a separate reader device. While the app had been available for Android users since 2015, it had just been released for the iPhone.
Also of significance was the announcement of the Freestyle Libre being made available to Medicare patients. The factory-calibrated system is the only CGM system recognized by Medicare that requires no user calibration whatsoever (either by fingerstick or manual data entry) at the time of the company’s announcement. The system also does not require the need for routine fingersticks and allows for patients to dose insulin based on the system’s results.