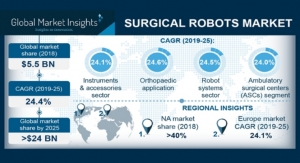
Premier Research04.19.19
Premier Research has added key expertise to its global CRO operations by naming three new medical device and diagnostics specialists.
Joining Premier Research are Vicki Gashwiler as executive director of clinical development services, medical device and diagnostics; and Sundeep Shrivastava as executive director of clinical development services, medical device and diagnostics.
To bolster the company’s regulatory expertise, John Pappan has been named director of regulatory affairs, medical device, and business strategy;
"These professionals inject years of experience to an already deep bench and speak to our commitment in the device space, which is part of our extensive 'built for biotech' services platform," said Dr. Mike Wilkinson, Premier Research chief operating officer. "John, Vicki, and Sundeep join an exemplary team that includes medical device experts Joanne Emmett and Janet Kube and regulatory expert Nach Davé, among our many other MDR and biotech/medtech talents."
Pappan, who joins Premier Research from a global regulatory compliance consultancy, has worked in regulatory affairs for more than a dozen years, including positions at DePuy Synthes (Johnson & Johnson), the Long Island University College of Pharmacy, Integra LifeSciences Corporation, ConMed Corporation, and mdi Consultants Inc. He earned a bachelor's degree in biology from Long Island University, a master's degree from Fordham University, and dual master's degrees from Long Island University, in drug regulatory affairs and pharmaceutical and healthcare administration.
"Premier Research is strengthening its service offerings to address the FDA's updated medical device lifecycle and regulatory pathway," said Nach Davé, vice president, development strategy. "John's help will be integral to our response to the challenges presented by the FDA's continued modernization of its approach to medical device safety, which include revisions to the 510(k) regulatory approval pathway. Premier Research can expect more sponsors—principally the small to midsize biotech innovators—to take advantage of the FDA's new breakthrough device designation rule for novel devices."
Davé noted the FDA published an updated guidance on Dec. 19, 2018, and announced changes to its 510(k) paradigm for medical device regulatory review and approval.
"Monitoring of medical device studies is also becoming more complex," said Joanne Emmett, vice president, medical devices, "as it becomes increasingly common for medical device studies to be spread across multiple clinical research centers around the globe. The strategic insights that Vicki and Sundeep will provide will be key to our success in the MDR space."
Gashwiler comes to Premier Research with nearly 20 years in healthcare, serving in clinical research positions at Abbott Vascular and the International Heart Institute of Montana. She has extensive experience in the therapeutic areas of cardiology, orthopedics, endocrinology, weight management, pulmonology, oncology, and vascular medicine, and is licensed and has served as a registered nurse in critical and cardiovascular care.
Gashwiler earned a bachelor's degree in nursing from Montana State University.
Shrivastava joins Premier Research with more than 20 years of extensive technology commercial experience in medical devices and CRO industry, holding prior clinical research leadership positions at IQVIA and Abbott Vascular. He earned his bachelor’s degree in statistics from the University of Bhopal and his MBA from the International Management Institute, New Delhi.
Premier Research, a mid-size clinical research company, is dedicated to helping biotech, specialty pharma, and device innovators transform life-changing ideas and breakthrough science into new medical treatments. As a global company, Premier Research specializes in the use of innovative technologies for smart study design and trial management to deliver clean, conclusive data to sponsors. Whether it's developing product lifecycle strategies, reducing clinical development cycle times, securing access to patients, navigating global regulations, maximizing the impact of limited rare disease data, or providing expertise in specific therapeutic areas, Premier Research is committed to helping its customers answer the unmet needs of patients across a broad range of medical conditions.
Joining Premier Research are Vicki Gashwiler as executive director of clinical development services, medical device and diagnostics; and Sundeep Shrivastava as executive director of clinical development services, medical device and diagnostics.
To bolster the company’s regulatory expertise, John Pappan has been named director of regulatory affairs, medical device, and business strategy;
"These professionals inject years of experience to an already deep bench and speak to our commitment in the device space, which is part of our extensive 'built for biotech' services platform," said Dr. Mike Wilkinson, Premier Research chief operating officer. "John, Vicki, and Sundeep join an exemplary team that includes medical device experts Joanne Emmett and Janet Kube and regulatory expert Nach Davé, among our many other MDR and biotech/medtech talents."
Pappan, who joins Premier Research from a global regulatory compliance consultancy, has worked in regulatory affairs for more than a dozen years, including positions at DePuy Synthes (Johnson & Johnson), the Long Island University College of Pharmacy, Integra LifeSciences Corporation, ConMed Corporation, and mdi Consultants Inc. He earned a bachelor's degree in biology from Long Island University, a master's degree from Fordham University, and dual master's degrees from Long Island University, in drug regulatory affairs and pharmaceutical and healthcare administration.
"Premier Research is strengthening its service offerings to address the FDA's updated medical device lifecycle and regulatory pathway," said Nach Davé, vice president, development strategy. "John's help will be integral to our response to the challenges presented by the FDA's continued modernization of its approach to medical device safety, which include revisions to the 510(k) regulatory approval pathway. Premier Research can expect more sponsors—principally the small to midsize biotech innovators—to take advantage of the FDA's new breakthrough device designation rule for novel devices."
Davé noted the FDA published an updated guidance on Dec. 19, 2018, and announced changes to its 510(k) paradigm for medical device regulatory review and approval.
"Monitoring of medical device studies is also becoming more complex," said Joanne Emmett, vice president, medical devices, "as it becomes increasingly common for medical device studies to be spread across multiple clinical research centers around the globe. The strategic insights that Vicki and Sundeep will provide will be key to our success in the MDR space."
Gashwiler comes to Premier Research with nearly 20 years in healthcare, serving in clinical research positions at Abbott Vascular and the International Heart Institute of Montana. She has extensive experience in the therapeutic areas of cardiology, orthopedics, endocrinology, weight management, pulmonology, oncology, and vascular medicine, and is licensed and has served as a registered nurse in critical and cardiovascular care.
Gashwiler earned a bachelor's degree in nursing from Montana State University.
Shrivastava joins Premier Research with more than 20 years of extensive technology commercial experience in medical devices and CRO industry, holding prior clinical research leadership positions at IQVIA and Abbott Vascular. He earned his bachelor’s degree in statistics from the University of Bhopal and his MBA from the International Management Institute, New Delhi.
Premier Research, a mid-size clinical research company, is dedicated to helping biotech, specialty pharma, and device innovators transform life-changing ideas and breakthrough science into new medical treatments. As a global company, Premier Research specializes in the use of innovative technologies for smart study design and trial management to deliver clean, conclusive data to sponsors. Whether it's developing product lifecycle strategies, reducing clinical development cycle times, securing access to patients, navigating global regulations, maximizing the impact of limited rare disease data, or providing expertise in specific therapeutic areas, Premier Research is committed to helping its customers answer the unmet needs of patients across a broad range of medical conditions.