Andrew DiMeo, Innovation & Design Coach at Trig09.06.19
Human-Factors Engineering (HFE) is gaining increasing attention, especially in the context of medical device product development. As technologies evolve, therapies advance, and devices become more complex, the role of HFE increases in priority. HFE is not new to medical devices; it first appeared in 1996 when it was added to the FDA’s Quality System Regulation. In the more than 20 years that followed, HFE has been included in a number of references by both regulatory bodies and standards organizations around the world.
What Exactly is Human-Factors Engineering?
Some recognized synonyms for HFE include ergonomics, human engineering, engineering psychology, and human-factors psychology. Each area of study has its own nuance, but there’s also much that’s common among these practices. The one book I recommend on this topic is “The Design of Everyday Things” by cognitive scientist Donald Norman. I have a Cliffs Notes version of this book in one sentence: “It’s not user error, it’s use error.”
When a product is improperly used, it is often users who take the blame. When a medical device company states that user error was the reason for a failure, well, that’s a good way to get the attention of the FDA. Norman’s book, originally published as “The Psychology of Everyday Things,” brings psychology and design together and puts the spotlight on the product itself as the culprit for failure.
This is especially important in medical devices where failures can result in harm to a user. In a commercial product, failure may result in losing a customer. In medical devices, failure may result in losing a life or limb. It’s serious business. It’s also smart business to do HFE.
HFE considers all the ways a user interacts with a product. This might include cognitive factors such as memory capacity, multitasking, and reaction time. It involves product design features that inform intended use. It takes into account ergonomic considerations such as variations in body size, range of motion, and strength.
The Role of Iterative Prototyping
In the 20-something years I’ve spent in roles from design engineer at a medical device company to professor in a college of engineering, I’ve witnessed a narrow definition for prototype. It is often described as a proof of concept with increasing fidelity over time. The prototype starts off as a bench-top experiment, then moves off the bench, and is refined over time as it gains a form factor asymptotically ever closer to a final commercial product.
Let’s consider a different perspective of prototyping as it plays a critical role in the research requirements of HFE. Specifically, this is called formative research. The design of such experiments is intended to gain user feedback that can be used to inform design features that reduce errors in product use—errors that are the fault of the product itself. When treating prototypes as described above, ever increasing resolution of the future product, the prototype we have today is not what we need to get the feedback we require for such a formative study. Indeed, we have already envisioned the future product and are working toward that.
As an example, let’s imagine we are working toward a handheld device that’s a point-of-care diagnostic. I may be interested in learning how a user may collect and read a sample. My current prototype is finally off the bench and is in the form of a functioning proof of concept. Rather than wowing the user that my awesome technology works, I get feedback that the device is too heavy, too big, and can’t be sterilized. Well, let’s say that, for this example, I already knew that. I have a form factor planned for the final device, we are just not there yet because of the current breadboard and large battery replacing the future solid-state chip and battery specified for the commercial product. As a result, worse than not wowing the future consumer, I got zero feedback regarding how the user may collect and read a sample.
At this point, I may be thinking the intended feedback won’t be possible until I get the prototype further refined. I’m going to need to spend more money and more time getting the electronics and battery further developed. Thus, a slow and expensive cycle of refining the prototype ensues. While we do want iteration in design, this cycle should not be confused with the iterative approach of design thinking.
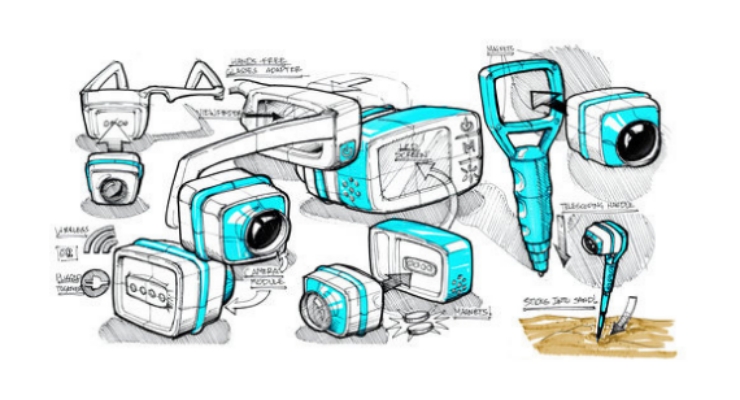
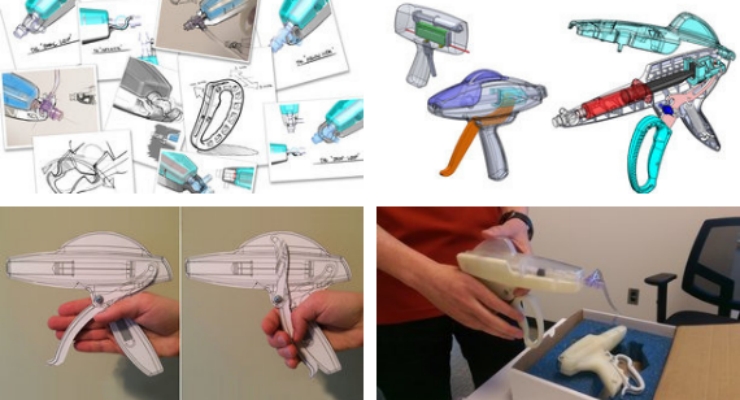
In this simplified example, I could have attained the desired feedback before I ever got the product off the bench. The formative research requirements of HFE often do not require a functional prototype. Rather, what the research requires is multiple prototypes representing multiple form factors, multiple sizes, and multiple shapes. Such non-functional prototypes can be valuable experimental instruments used to gain insights on how the user may interact with the device.
Given the example, imagine an early research finding that indicates users prefer a larger product that can stand alone on a cart with other equipment they are concurrently interacting with. How might that research finding alter the course of product development?
Rapid, Parallel, and Iterative Prototyping—and the Technology Driving It
Such feedback can be gained early in the design process when armed with a toolbox of prototyping methods and technologies. These may include low-resolution sketches and storyboards, 2D models (such as from laser cutting), and 3D models using the array of additive manufacturing techniques allowing for myriad design features that impact use from color to material finish.
Consider rapid, parallel, and iterative methods. Ask yourself, “What is the fastest way to get the feedback I want to inform the design today?” The parallel nature of prototyping means having more than one model to investigate user preference, ergonomics, misuse, etc.
Be able to ask, “Of these four storyboards, rank each process from most to least desirable. Why?” or, “Please play with these 10 grip designs…,” which have been made with a 3D printer, while observing how they are handled by the user, getting them to rank order each, and asking why.
Returning to the example, rather than spending time and money getting the working prototype off of the bench, early prototyping is being used to collect critical feedback that will both reduce use error as required by HFE, and also increase user preference of your product. Significant technical burdens that may be impacted by form factor, such as the solid-state electronics and battery configuration, could have been alleviated up front. Or perhaps, they could have been identified early as a critical risk to the project.
Instead of thinking about a prototype as the current best version of a pre-production product, think of many prototypes to get feedback for a current question.
Imagine that a year from now I’m going on a week-long camping trip where I’ll be fly fishing every day. Over the year leading up to the trip, I can slowly attain ever increasing quality of equipment. A nice new fly rod and giant supply of line and flies are assembled for the trip. The problem is, while I’m finally out there camping in the remote mountains, the fish aren’t biting the flies I chose to bring.
The better approach may by going on multiple small fishing trips over the year. Bring dozens and dozens of flies, try different lines, and sample multiple rods by visiting Orvis more than once. Trying lots of flies may reveal that there’s five or six that really work well for me, my style, and the fish I’m interested in. Different rods with different lengths, weights, and grip styles will all impact my results. When I finally do go on that trip, all the formative testing I’ve done will pay off while in the water.
Formative feedback for HFE requires taking multiple prototypes to the user for them to try in the context of their everyday work, and rank order the feedback on multiple factors such as desirability, ergonomics, cognitive aspects, etc. Of utmost importance is the feedback on intended use that reduces use error.
Andrew DiMeo is innovation & design coach at Trig, a Chapel Hill, NC-based full-service industrial design firm serving the consumer, healthcare, and durable goods markets. DiMeo’s experience includes New York City Motion Picture set design and 20-plus years in medical innovation, including 12 years on the faculty at the University of North Carolina and North Carolina State University in Biomedical Engineering. His students’ coursework led to multiple startups such as 410 Medical and winning national competitions from NCEES, NIH, and VentureWell. He was a founder of the North Carolina Medical Device Organization, EG-Gilero, and Novocor Medical; design engineer at Alaris Medical Systems; business advisor for the Wallace H. Coulter Foundation; advisor to the NIH C3i Program; director of Duke NeuroInnovations; and on the planning team for BME Idea.
What Exactly is Human-Factors Engineering?
Some recognized synonyms for HFE include ergonomics, human engineering, engineering psychology, and human-factors psychology. Each area of study has its own nuance, but there’s also much that’s common among these practices. The one book I recommend on this topic is “The Design of Everyday Things” by cognitive scientist Donald Norman. I have a Cliffs Notes version of this book in one sentence: “It’s not user error, it’s use error.”
When a product is improperly used, it is often users who take the blame. When a medical device company states that user error was the reason for a failure, well, that’s a good way to get the attention of the FDA. Norman’s book, originally published as “The Psychology of Everyday Things,” brings psychology and design together and puts the spotlight on the product itself as the culprit for failure.
This is especially important in medical devices where failures can result in harm to a user. In a commercial product, failure may result in losing a customer. In medical devices, failure may result in losing a life or limb. It’s serious business. It’s also smart business to do HFE.
HFE considers all the ways a user interacts with a product. This might include cognitive factors such as memory capacity, multitasking, and reaction time. It involves product design features that inform intended use. It takes into account ergonomic considerations such as variations in body size, range of motion, and strength.
The Role of Iterative Prototyping
In the 20-something years I’ve spent in roles from design engineer at a medical device company to professor in a college of engineering, I’ve witnessed a narrow definition for prototype. It is often described as a proof of concept with increasing fidelity over time. The prototype starts off as a bench-top experiment, then moves off the bench, and is refined over time as it gains a form factor asymptotically ever closer to a final commercial product.
Let’s consider a different perspective of prototyping as it plays a critical role in the research requirements of HFE. Specifically, this is called formative research. The design of such experiments is intended to gain user feedback that can be used to inform design features that reduce errors in product use—errors that are the fault of the product itself. When treating prototypes as described above, ever increasing resolution of the future product, the prototype we have today is not what we need to get the feedback we require for such a formative study. Indeed, we have already envisioned the future product and are working toward that.
As an example, let’s imagine we are working toward a handheld device that’s a point-of-care diagnostic. I may be interested in learning how a user may collect and read a sample. My current prototype is finally off the bench and is in the form of a functioning proof of concept. Rather than wowing the user that my awesome technology works, I get feedback that the device is too heavy, too big, and can’t be sterilized. Well, let’s say that, for this example, I already knew that. I have a form factor planned for the final device, we are just not there yet because of the current breadboard and large battery replacing the future solid-state chip and battery specified for the commercial product. As a result, worse than not wowing the future consumer, I got zero feedback regarding how the user may collect and read a sample.
At this point, I may be thinking the intended feedback won’t be possible until I get the prototype further refined. I’m going to need to spend more money and more time getting the electronics and battery further developed. Thus, a slow and expensive cycle of refining the prototype ensues. While we do want iteration in design, this cycle should not be confused with the iterative approach of design thinking.
In this simplified example, I could have attained the desired feedback before I ever got the product off the bench. The formative research requirements of HFE often do not require a functional prototype. Rather, what the research requires is multiple prototypes representing multiple form factors, multiple sizes, and multiple shapes. Such non-functional prototypes can be valuable experimental instruments used to gain insights on how the user may interact with the device.
Given the example, imagine an early research finding that indicates users prefer a larger product that can stand alone on a cart with other equipment they are concurrently interacting with. How might that research finding alter the course of product development?
Rapid, Parallel, and Iterative Prototyping—and the Technology Driving It
Such feedback can be gained early in the design process when armed with a toolbox of prototyping methods and technologies. These may include low-resolution sketches and storyboards, 2D models (such as from laser cutting), and 3D models using the array of additive manufacturing techniques allowing for myriad design features that impact use from color to material finish.
Consider rapid, parallel, and iterative methods. Ask yourself, “What is the fastest way to get the feedback I want to inform the design today?” The parallel nature of prototyping means having more than one model to investigate user preference, ergonomics, misuse, etc.
Be able to ask, “Of these four storyboards, rank each process from most to least desirable. Why?” or, “Please play with these 10 grip designs…,” which have been made with a 3D printer, while observing how they are handled by the user, getting them to rank order each, and asking why.
Returning to the example, rather than spending time and money getting the working prototype off of the bench, early prototyping is being used to collect critical feedback that will both reduce use error as required by HFE, and also increase user preference of your product. Significant technical burdens that may be impacted by form factor, such as the solid-state electronics and battery configuration, could have been alleviated up front. Or perhaps, they could have been identified early as a critical risk to the project.
Instead of thinking about a prototype as the current best version of a pre-production product, think of many prototypes to get feedback for a current question.
Imagine that a year from now I’m going on a week-long camping trip where I’ll be fly fishing every day. Over the year leading up to the trip, I can slowly attain ever increasing quality of equipment. A nice new fly rod and giant supply of line and flies are assembled for the trip. The problem is, while I’m finally out there camping in the remote mountains, the fish aren’t biting the flies I chose to bring.
The better approach may by going on multiple small fishing trips over the year. Bring dozens and dozens of flies, try different lines, and sample multiple rods by visiting Orvis more than once. Trying lots of flies may reveal that there’s five or six that really work well for me, my style, and the fish I’m interested in. Different rods with different lengths, weights, and grip styles will all impact my results. When I finally do go on that trip, all the formative testing I’ve done will pay off while in the water.
Formative feedback for HFE requires taking multiple prototypes to the user for them to try in the context of their everyday work, and rank order the feedback on multiple factors such as desirability, ergonomics, cognitive aspects, etc. Of utmost importance is the feedback on intended use that reduces use error.
Andrew DiMeo is innovation & design coach at Trig, a Chapel Hill, NC-based full-service industrial design firm serving the consumer, healthcare, and durable goods markets. DiMeo’s experience includes New York City Motion Picture set design and 20-plus years in medical innovation, including 12 years on the faculty at the University of North Carolina and North Carolina State University in Biomedical Engineering. His students’ coursework led to multiple startups such as 410 Medical and winning national competitions from NCEES, NIH, and VentureWell. He was a founder of the North Carolina Medical Device Organization, EG-Gilero, and Novocor Medical; design engineer at Alaris Medical Systems; business advisor for the Wallace H. Coulter Foundation; advisor to the NIH C3i Program; director of Duke NeuroInnovations; and on the planning team for BME Idea.