Jeff Barrett, President and CEO, J-Pac Medical03.20.18
There is a lot of excitement in the medical device packaging industry about pre-validated packaging. Using pre-validated packaging may reduce time to market and minimize expense related to package development and validation. However, international standards defining medical device and healthcare packaging systems’ validation requirements are complex and involve several levels of materials selection, design qualification, and process validation, as well as design controls. Pre-validated packaging may not comply with all the requirements depending on the specific device involved. Nevertheless, pre-validated packaging can be a useful strategy if properly implemented by the sterile packaging manufacturer (SPM) and device packager within the framework of these standards. The purpose of this article is to clarify how medical device manufacturers (MDMs) can ensure their pre-validated packaging systems will meet these requirements.
Packaging Standards for Terminally Sterilized Devices
Sterile packaging systems need to ensure the sterility of their contents until they are opened for use. The sterile barrier system (SBS) must also be designed to ensure aseptic presentation at the point of use. ISO 11607 (part 1 and part 2) is the global standard governing packaging for terminally sterilized medical devices. ISO 11607-1 defines requirements for SBS materials selection, and their design and testing. ISO 11607-2 defines manufacturing critical process validation requirements for forming, sealing, and the assembly processes. Both parts of ISO 11607 were designed to meet the Essential Requirements of the European Medical Device Directives, making them requirements for MDMs in the EU or companies selling into the EU.
Key Definitions
One of the most important parts of ISO 11607 is providing a list of clear terminology used for medical device and healthcare packaging. There are a few very important definitions that are used in the standards that define the nuances of a packaging system, which is comprised of several components.
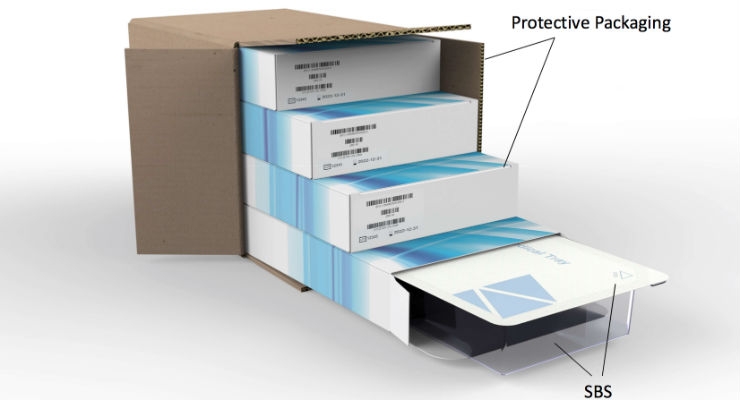
Sterile Barrier System (SBS): The minimum package that minimizes the risk of ingress of microorganisms and allows aseptic presentation of the sterile contents at the point of use. For example, a thermoformed tray with a Tyvek lid can be an SBS. Header bags and Chevron pouches are common sterile barrier systems.
Protective Packaging: The configuration of materials designed to prevent damage to the sterile barrier system and its contents from the time of their assembly until the point of use. Examples include corrugated boxes, dunnage, or additional packaging inside a sealed tray or pouch that prevents the medical device from damaging the sterile barrier system. For example, while a package design can be tested to ensure it maintains a sterile barrier, the enclosed device must not damage the SBS during shipping and handling. This is usually demonstrated by simulated distribution testing of the packaging system.
Preformed Sterile Barrier System: A sterile barrier system that is supplied partially assembled for filling and final closure or sealing. Examples of these include porous and nonporous Chevron pouches, as well as header and patch bags. These are typically purchased from packaging suppliers partially assembled and used during final packaging by making a final closure seal. While a thermoformed tray does not technically constitute a pre-formed sterile barrier system, the tray manufacturing process should be validated.
Packaging System: The combination of the sterile barrier system and protective packaging. The key concept here is that both the device and the sterile barrier must be protected from damage
Applying the Definitions
The combination of the sterile barrier system and the protective packaging forms the packaging system. The entire packaging system must be designed to protect both the device and the sterile barrier itself during shipping, handling and storage. A common cause of packaging failure occurs because the device inside the package creates forces during shipping that can compromise the sterile barrier materials or seals. Because these forces are unique to the specific device, it is unlikely pre-validated packaging can negate the need for physical and climactic stress testing data for the device in question.
How Is Sterile Packaging Validated?
ISO 11607 highlights the major requirements that must be accomplished to properly validate terminally sterilized medical device packaging. Following are several of the major highlights of those requirements.
Data Required for Pre-Validated Packaging
It is critical to remember that all tests must be completed on the entire packaging system for the specific device in question. Data developed for a “worst case” for a family of similar products may be sufficient under certain circumstances.
MDMs should ask several important questions of their SPMs or contract packager to ensure they have the correct documentation for their design history file.
Conclusion
Pre-validated sterile packaging is a novel concept that, if properly executed, can help MDMs reduce expense and improve time to market. However, the ISO 11607 standard (Parts 1 and 2) is complex and cites three key components of a “validated” packaging system.
The MDM’s unique devices, sterilization cycle, distribution environment, and manufacturing processes often require pre-validated packaging solutions to undergo additional qualification and validation testing to satisfy regulatory requirements. MDMs should ask specific questions to understand how pre-validated packaging meets these requirements and clearly identify any additional testing that may be required. Finally, MDMs must ensure that all validation data is maintained in their design history files, as they are ultimately responsible.
Additional Resources
Jeff Barrett is the president and CEO of J-Pac Medical—a manufacturing outsourcing partner to leading medical device companies. He has more than 25 years of experience building high-growth medical device companies from initial funding through IPO. Barrett held CEO positions at GI Supply and Optim LLC, as well as vice president of operations roles at Haemonetics and Aspect Medical Systems (acquired by Covidien). He holds an MBA from Boston University’s Graduate School of Management and a B.S. in industrial engineering and B.A. in economics from Rutgers University.
Packaging Standards for Terminally Sterilized Devices
Sterile packaging systems need to ensure the sterility of their contents until they are opened for use. The sterile barrier system (SBS) must also be designed to ensure aseptic presentation at the point of use. ISO 11607 (part 1 and part 2) is the global standard governing packaging for terminally sterilized medical devices. ISO 11607-1 defines requirements for SBS materials selection, and their design and testing. ISO 11607-2 defines manufacturing critical process validation requirements for forming, sealing, and the assembly processes. Both parts of ISO 11607 were designed to meet the Essential Requirements of the European Medical Device Directives, making them requirements for MDMs in the EU or companies selling into the EU.
Key Definitions
One of the most important parts of ISO 11607 is providing a list of clear terminology used for medical device and healthcare packaging. There are a few very important definitions that are used in the standards that define the nuances of a packaging system, which is comprised of several components.
Sterile Barrier System (SBS): The minimum package that minimizes the risk of ingress of microorganisms and allows aseptic presentation of the sterile contents at the point of use. For example, a thermoformed tray with a Tyvek lid can be an SBS. Header bags and Chevron pouches are common sterile barrier systems.
Protective Packaging: The configuration of materials designed to prevent damage to the sterile barrier system and its contents from the time of their assembly until the point of use. Examples include corrugated boxes, dunnage, or additional packaging inside a sealed tray or pouch that prevents the medical device from damaging the sterile barrier system. For example, while a package design can be tested to ensure it maintains a sterile barrier, the enclosed device must not damage the SBS during shipping and handling. This is usually demonstrated by simulated distribution testing of the packaging system.
Preformed Sterile Barrier System: A sterile barrier system that is supplied partially assembled for filling and final closure or sealing. Examples of these include porous and nonporous Chevron pouches, as well as header and patch bags. These are typically purchased from packaging suppliers partially assembled and used during final packaging by making a final closure seal. While a thermoformed tray does not technically constitute a pre-formed sterile barrier system, the tray manufacturing process should be validated.
Packaging System: The combination of the sterile barrier system and protective packaging. The key concept here is that both the device and the sterile barrier must be protected from damage
Applying the Definitions
The combination of the sterile barrier system and the protective packaging forms the packaging system. The entire packaging system must be designed to protect both the device and the sterile barrier itself during shipping, handling and storage. A common cause of packaging failure occurs because the device inside the package creates forces during shipping that can compromise the sterile barrier materials or seals. Because these forces are unique to the specific device, it is unlikely pre-validated packaging can negate the need for physical and climactic stress testing data for the device in question.
How Is Sterile Packaging Validated?
ISO 11607 highlights the major requirements that must be accomplished to properly validate terminally sterilized medical device packaging. Following are several of the major highlights of those requirements.
- Packaging materials must be selected and documented within a quality system based on the requirements of the device and sterilization method used. These include adequacy of the microbial barrier, biocompatibility and toxicity attributes, sterilization effects, sealing effectiveness, compatibility of the device and SBS materials that contact each other, and compatibility with labeling. All of these are met while meeting customer usage requirements.
- It must be proven that the sterile barrier system materials and seals between them maintain sterility over time. This involves a post-sterilization stability study for the claimed shelf life of the finished product. Changes to sterile barrier system materials may necessitate repeating previous testing, making it critical to ensure pre-validated packaging materials are documented in the design history file. Similarly, the same or more severe and demanding sterilization cycle required for the specific device must be used.
- The process used to seal the sterile barrier system must be validated. This requires a critical manufacturing process validation, which includes an IQ, OQ, and PQ on the sealing equipment performed at the manufacturing location.
- Packaging system design qualification testing must be conducted on the packaging system. The major objectives are to ensure that (a) the sterile barrier system is not damaged during transportation and handling and maintains integrity, and (b) the specific medical device(s) contained in the SBS is not compromised. This is achieved by conducting post-sterilization climatic and shipping simulation followed by evaluation and testing of both the sterile barrier and the functionality of the device as well as the rest of the packaging components. Pre-validated packaging cannot typically cover this requirement because each device and resultant packaging system is unique.
Data Required for Pre-Validated Packaging
It is critical to remember that all tests must be completed on the entire packaging system for the specific device in question. Data developed for a “worst case” for a family of similar products may be sufficient under certain circumstances.
MDMs should ask several important questions of their SPMs or contract packager to ensure they have the correct documentation for their design history file.
- Do you provide us data showing that the sterile barrier system materials and seals are validated for the claimed shelf life? How have you proven that the sterile barrier system and its components are compatible with the specific sterilization cycle we require? Is the data adequate for our regulatory filing?
- Do you provide us with data showing that the process to seal the preformed sterile barrier system or the SBS components is validated? Note, if the packaging supplier is not performing the sealing function then the MDM or contract packager must conduct a sealing validation for the specific SBS and equipment used.
- How do we support the claim that the packaging system is adequate to protect the sterile barrier as well as our medical device during shipping and handling? What rationale is documented that our device will be adequately protected and will not cause damage to the sterile barrier system during handling? The only way to claim pre-validated packaging in this case is for the SPM or packager to have tested similar products sterilized in the same modality that could be used as rationale to justify not repeating the test for the MDM’s specific product. There is some risk for the MDM that their regulatory body may reject this rationale on the grounds that the MDM’s device is unique and cannot be included in a “family” of similar products. In this case, the packaging system design qualification performance testing must be repeated.
Conclusion
Pre-validated sterile packaging is a novel concept that, if properly executed, can help MDMs reduce expense and improve time to market. However, the ISO 11607 standard (Parts 1 and 2) is complex and cites three key components of a “validated” packaging system.
- The design and development of the packaging system, which includes material selection, design, and packaging system design qualification testing
- Qualifying, validating, or providing proof of the stability of the SBS materials and seals
- SBS sealing process validation
The MDM’s unique devices, sterilization cycle, distribution environment, and manufacturing processes often require pre-validated packaging solutions to undergo additional qualification and validation testing to satisfy regulatory requirements. MDMs should ask specific questions to understand how pre-validated packaging meets these requirements and clearly identify any additional testing that may be required. Finally, MDMs must ensure that all validation data is maintained in their design history files, as they are ultimately responsible.
Additional Resources
- ISO 11607-1:2006/(R) 2010 Packaging for terminally sterilized medical devices—Part 1: Requirements for materials, sterile barrier systems, and packaging systems
- ISO 11607-1: 2006/A1: 2014 Packaging for terminally sterilized medical devices—Part 1: Requirements for materials, sterile barrier systems, and packaging, Amendment 1
- ISO 11607-2:2006/(R) 2010 Packaging for terminally sterilized medical devices—Part 2: Validation requirements for forming, sealing and assembly processes
- ISO 11607-2: 2006/A1: 2014 Packaging for terminally sterilized medical devices—Part 2: Validation requirements for forming, sealing, and assembly processes, Amendment 1
- ANSI/AAMI/ISO TIR16775: 2014, Technical Information Report, Packaging for terminally sterilized medical devices-Guidance on the application of ISO 11607-1 and ISO 11607-2
Jeff Barrett is the president and CEO of J-Pac Medical—a manufacturing outsourcing partner to leading medical device companies. He has more than 25 years of experience building high-growth medical device companies from initial funding through IPO. Barrett held CEO positions at GI Supply and Optim LLC, as well as vice president of operations roles at Haemonetics and Aspect Medical Systems (acquired by Covidien). He holds an MBA from Boston University’s Graduate School of Management and a B.S. in industrial engineering and B.A. in economics from Rutgers University.